
Conjugation Reaction between Citronellal and L-Tyrosine and Its
Antimicrobial Properties against Bacteria and Fungi
1
Magister Program, Department of Chemistry, Universitas Syiah Kuala, Banda Aceh, Indonesia
2
Department of Chemistry, Universitas Syiah Kuala, Banda Aceh, Indonesia
3
Faculty of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
4
Department of Cardiovascular, Dr. Zainoel Abidin Hospital, Banda Aceh, Indonesia
5
Department of Chemistry, Universitas Tadulako, Palu, Sulawesi Tengah, Indonesia
6
Herbal Research Centre, Universitas Yarsi, Jakarta, Indonesia
Department of Chemical Engineering, Universitas Syiah Kuala, Banda Aceh, Indonesia
8
PUI-Nilam Aceh-Atsiri Research Centre, Universitas Syiah Kuala, Banda Aceh, Indonesia
9
Departement of Pharmacy, Universitas Syiah Kuala, Banda Aceh, Indonesia
10
Pusat Riset Obat Herbal, Universitas Syiah Kuala, Banda Aceh, Indonesia
11
Pusat Riset Etnoscience, Universitas Syiah Kuala, Banda Aceh, Indonesia
Keywords: Conjugation Reaction, Citronellal, L-tyrosine, Staphylococcus aureus, Escherichia coli, Candida
albicans
.
Abstract: The study of citronellal with L-tyrosin conjugation for antimicrobial properties has been
conducted. The aim of this study to determine the relationship stucture between two compounds
citronellal and L-tyrosine on antimicrobial activity against Staphylococcus aureus, Escherichia
coli, and Candida albicans. The conjugation product obtained was yellow-white solid amorphous
with the Rf value was 0.84 and the percentage of yield was 71.12%. The FT-IR spectra peak at
3205.69 cm
-1
is represented the N-H stretching vibration from L-tyrosine, while the spectra appears
at 1460.11 - 1438.90 cm
-1
are represented the C=N which derived from imine or immonium from
shift base reaction between citronellal and L-tyrosine. The GC-MS analysis showed that the peak
15 observed at RT 10.27 min. be expected a conjugation product with the m/z 316 [M+H]
+
ion.
The antimicrobial activity were determined by well diffusion method and the results showed that
product of conjugation were have no antimicrobail activities at concentration tested.
1 INTRODUCTION
Citronellal is a monoterpene that has two optical
isomers with a molecular weight of 154.25 g / mol.
The reactivity of citronellal is resulting from carbonyl
group, double bond, and acidity of Hα. These groups
allowing citronellal to react with an acid or a base.
Some biological activities of citronellal including
insecticides (Griffith and Grentile, 1979), perfumery
(Anderson et al., 1993; Sangwan et al., 2001),
stimulants, antidepressants, analgesics, antipyretics,
and antimicrobials (Adhikari et al., 2015) L-tyrosine
or 4-hydroxyphenylalanine is a non-essential amino
acid, a primary amine, has a polar group. L-tyrosine
widely used in food industry and pharmaceutical
industry (Tina, 2007). The conjugation of natural
product with several constituents has attracted some
researchers due to their biological reactivity on
several microorganisms and cells (Martinez et al.,
2015; Hong et al., 2017). For examples, novobiocin,
serrulatane, xanthorhizol (Finland and Nichols, 1957;
Lewis and Klibanov, 2005; Rukayadi and Hwang,
2006), which contain prenil and aromatic hydroxy
groups are believed to play an important role in
antimicrobial activity.
2,4-Dimethyl-2,6-heptadiene-1-ol and 5-Amino-
2-methylphenol are two compounds produced from a
conjugation reaction are known to have antibacterial
activity against Staphylococcus epidermidis (Ys,
2015), Rusdin reported that product conjugation
between citronellal and L-tyrosine has antibacterial
Rila Suryani
1
, Nazaruddin
2
, Kartini Hasballah
3
, Muhammad Diah
3,4
, Hardi Yusuf
5
, Juniarti
6
,
Syaifullah Muhammad
7,8
, Khairan
8,9,10,11
74
Suryani, R., Nazaruddin, N., Hasballah, K., Diah, M., Yusuf, H., Juniarti, ., Muhammad, S. and Khairan, .
Conjugation Reaction between Citronellal and L-Tyrosine and Its Antimicrobial Properties against Bacteria and Fungi.
DOI: 10.5220/0009957000740079
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 74-79
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

activity against Stapylococcus aureus (Al-Garawi et
al., 2012). The computational analysis result showed
the formation of imine conjugation bonds (imine fond
formation) between the citronellal (3,7-dimethyl-6-
octenal) with the amino acid L-tyrosine (Rusdin et al.,
2018). However, the research conducted by Rusdin
and Hardi has not been able to determine the type of
conjugate product. In this study, we interest to
determine product of conjugation obtained by GC-
MS spectroscopy and evaluate its activity against
Staphylococcus aureus, Escherichia coli, and
Candida albicans.
2 EXPERIMENTAL
2.1 Materials
Citronellal and L-tyrosine were received from
Department of Chemistry, Universitas Tadulako,
Palu, Sulawesi Tengah, Indonesia. Pentane hexane,
ethyl acetate, methanol, potassium hydroxide (KOH),
diethyl ether, 96% ethanol, distilled water,
physiological NaCl, and dimethyl sulfoxide (DMSO)
were obtained from Department of Pharmacy,
Universitas Syiah Kuala, Banda Aceh, Indonesia.
2.2 Conjugation Reaction Citronellal
and L-Tyrosine
The conjugation reaction between citronellal and L-
tyrosine was conducted by Al-Gharawi and Rusdin
methods (Al-Garawi et al., 2012; Rusdin et al., 2018).
Briefly, 0.18 grams of citronellal (1.2 mmol) in 10
mL of methanol was reacted with 0.18 grams of L-
tyrosine (1 mmol) in 15 mL of methanol, then 0.056
grams of KOH was added. The mixture then refluxed
for 8 hours at 60ºC. The conjugate (product) then
concentrated using rotary evaporator and washed
three times with pure ethanol. The product washed
again with diethyl ether and evaporated at room
temperature to obtain yellow-white solid amorphous.
2.3 Column Chromatography
Purification of conjugation product obtained was
conducted by column chromatography using silica
gel F
250
as stationary phase. As mobile phase, we used
a mixture of hexane: ethyl acetate (9:1). The collected
fractions were submitted into thin-layer
chromatography (TLC), using mobile phase a mixture
of hexane: ethyl acetate (9:1), and the chromatogram
was observed using UV-lamp at 250 nm. The major
fraction obtained then analysis by GC-MS
spectroscopy.
2.4 FT-IR Analysis
The FT-IR analysis spectrophotometer was
performed at wave numbers 4000-500 cm-1 using
Schimadzu model.
2.5 GC-MS Analysis
The conjugation product and collected fraction were
analysed by gas chromatography-mass spectroscopy
6890 equipped with capillary column Agilent HP 5
MS (60 x 0.25 x 0.25). The operating condition of the
gas chromatography were 1.0 ml/min (He), with
volume injection was 0.5µl. Oven temperature 300ºC
for 40 min.
2.6 Antimicrobial Activity
The antifungal activity was determined by Kirby-
Bauer method. The sterile Sabouraud's Dextrose Agar
(SDA) media was poured into petri dish and allowed
to solidify. The strains of Candida albicans was
spread out on the solidified media of SDA by using
the sterile cotton bud. The paper disc was laid out on
the surface of the agar medium. To each of disc 12 µl
of negative control (solvent), positive control
(nystatine), and tested compound and was loaded and
subsequently incubated at 37ºC for 48 hours. In the
same procedure, the antibacterial activity of the tested
compounds against Staphylococcus aureus and
Escherichia coli were performed using Mueller
Hinton Agar (MHA) media and subsequently
incubated at 37ºC for 24 and 48 hours. In the
antibacterial assay we used ciprofloxacin and
gentamycin as positive controls for Staphylococcus
aureus and Escherichia coli respectively. Then, the
inhibition effect of the tested compunds were
determined. The antimicrobial activities were
performed in triplicates.
3 RESULTS AND DISCUSSION
3.1 Conjugation Reaction Citronellal
and L-Tyrosine
Conjugation reaction between citronellal and L-
tyrosine were used potassium hydroxide (KOH) as a
catalyst. Ritter mentioned that carbonyl group, double
bond, and Hα atom from citronellal allows to react
with an acid or base. L-tyrosine is a primary amine, a
Conjugation Reaction between Citronellal and L-Tyrosine and Its Antimicrobial Properties against Bacteria and Fungi
75
base, is readily react to citronellal (an aldehyde)
(Griffith and Grentile, 1979). Murray mentioned that
the reaction between an aldehyde or a ketone with a
primary amine will produce an imine through a shift
base mechanism. L-tyrosine act as a nucleophilic, and
a citronellal act as electrophilic to form an immonium
salt, through the mechanism of base shift reaction,
this readily to form an imine compound (R
2
C=NR)
(Murray, 2010). The mechanism reaction of imine
formation from primary amine and an aldehyde
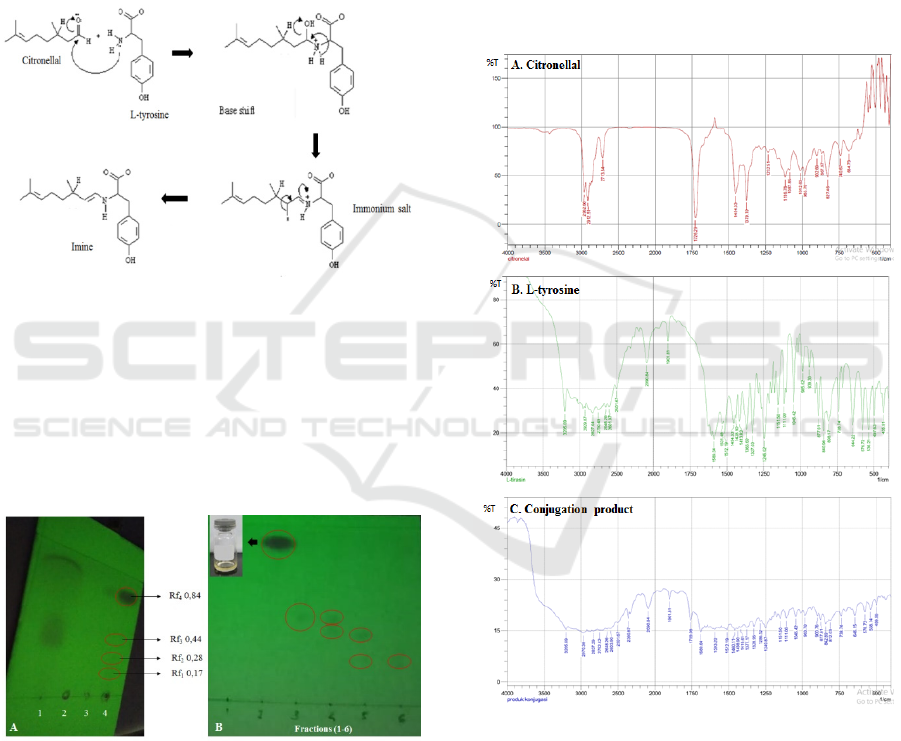
shown in Figure 1 below.
Figure 1: The mechanism reaction of imine formation from
citronellal and L-tyrosine.
The obtained product was white-yellow in colour and
amorphous in shape. The fragrant of the product was
lighter than citronellal. The percentage yield of
product was 71.12% or 0.51 gram. The TLC result
showed that the product has four bands with the Rf
values were 0.17; 0.28; 0.44; and 0.84 (Figure 2A).
Figure 2A: TLC result of the conjugation product: 1.
Citronellal; 2. L-tyrosine; 3. Citronellal + L-tyrosine; and 4.
Conjugation product: B. TLC result of the conjugation
product from column chromatography using mobile phase
a mixture of hexane: ethyl acetate (9:1), and the
chromatogram of the fractions was observed using UV-
lamp at 250 nm.
3.2 FT-IR Analysis
The FT-IR analysis of citronellal, L-tyrosine, and
conjugation product shown below. Figure 3A showed
that the spectra at 1729 cm
-1
indicated the presence of
carbonyl groups (-C=O), while the absorption at 2913
cm
-1
and 1423 cm
-1
were represented of functional
group of C-H and C=N. All these functional group are
typical for citronellal. Fig. 3B pointed that the
absorption at 1589 cm
-1
, 3363 cm
-1
and 1242 cm
-1
were indicated the presence of aromatic functional
group of C=C, N-H, and C-N respectively. These
functional groups are typical for L-tyrosine.
Figure 3: The FT-IR analysis of A. Citronellal; B. L-
tyrosine; C. Conjugation product.
The FT-IR analysis of the conjugation product
showed strong absorption of N-H groups at 3205.69
cm
-1
(Figure 3C). Murray and Silverstain mentioned
that the absorption of C=O carbonyl appears at
1759.08 cm
-1
with low intensity. The absorption of
C=C alkenes appears at 1669.64 cm
-1
and the
spectrum of C=C aromatic absorb at 1512.19 cm
-1
.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
76
While the C=N spectrum appears at 1460.11-1438.90
cm
-1
(Murray, 2010; Silverstein et al., 2005).
3.3 GC-MS Analysis
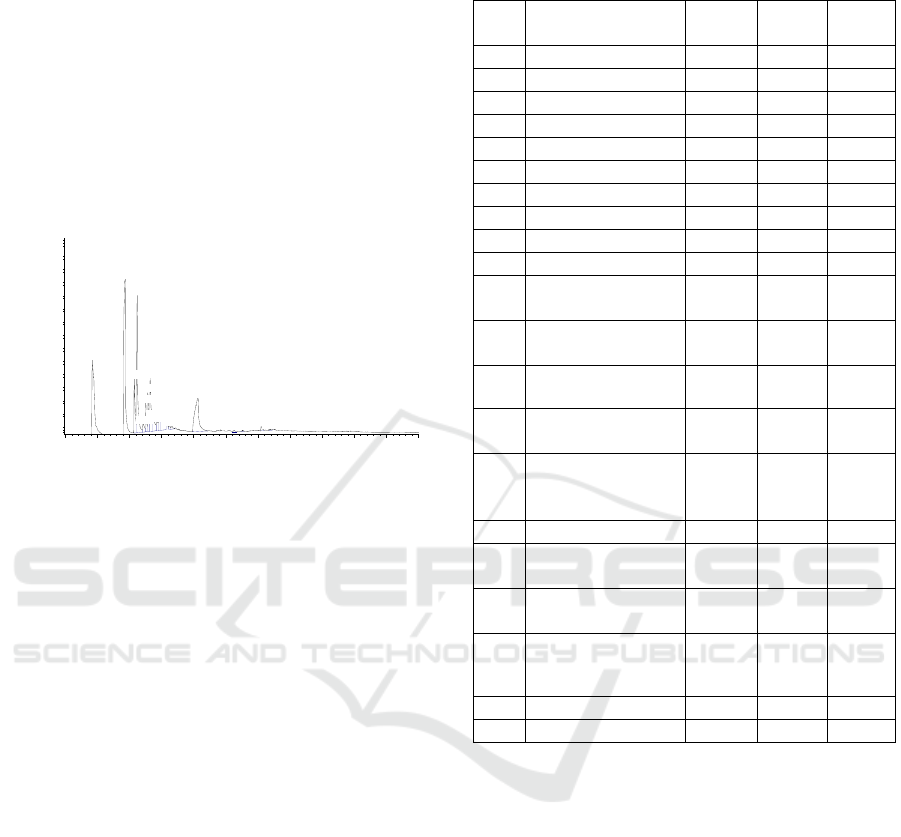
The major fraction (fraction 2, Figure 2B) obtained
from column chromatography with the Rf value of
0.86 that to be expected conjugation product was
analysis by GC-MS. The GC spectral of fraction 2 is
presented in Figure 4.
Figure 4: The GC analysis of fraction 2 isolated from
conjugation product.
The composition of fraction 2 isolated from
conjugation product is shown in Table 2. The profile
of fraction 2 from conjugation product contains 20
compounds. The main compound of the fraction 2 is
phenolic compound. The major of phenolic
compounds in fraction 2 was p-cresol (13.20%) and
L-Tyrosine and Octanal,7-hydroxy-3,7-dimethyl
(12.92%). The other phenolic compounds in
moderate percentage were assigned to 3-propyl-
phenol (2.3%), 3-ethyl-phenol (2.49%), 2,4-
dimethylphenol (4.14%), 4-ethyl-2-methyl-phenol
(4.53%), 4-ethylphenol (5.99%), and Octanal,7-
hydroxy-3,7-dimethyl (0.53%).
The Ritter stated that L-tyrosine (a primary
amine) is readily react to citronellal (an aldehyde) to
form an imine compound (conjugation product)
through a shift base mechanism (Figure 1) (Griffith
and Grentile, 1979). The characterization of the
product conducted by GC-MS, to identify the
conjugation product between citronellal and L-
tyrosine. The MS characterization of the product is
shown in Figure 5, and be expected observed at RT
10.27 with the percentage area of the peak of 12.92%
(peak 15).
Table 1: The GC-MS analysis of fraction 2 isolated from
conjugation product.
No. Name
RT
(min)
%
Area
m/z
1. Carboxylic acid 3.70 16.47 44.0
2. Phenol 5.74 25.35 94.1
3. Hydroxytoluene 6.31 5.34 108.14
4. p-cresol 6.49 13.20 108.14
5. 2,3-dimetyl-phenol 6.87 1.44 122.16
6. 3-ethyl-phenol 7.03 2.49 122.16
7. 2,4-dimethylphenol 7.15 4.14 122.16
8. 4-ethylphenol 7.30 5.99 122.16
9. 3-propyl-Phenol 7.50 2.30 136.19
10. 2-propyl-Phenol 7.73 1.19 139.19
11. 2-ethyl-4-methyl-
Phenol
7.86 2.27 137.01
12. 4-ethyl-2-methyl-
Phenol
7.97 4.53 137.01
13.
2,3-Dimethyl-N-
phenylalanine
8.50 0.49 197.00
14. Octanal,7-hydroxy-
3,7-dimethyl
8.63 0.57 154.00
15. L-Tyrosine and
Octanal,7-hydroxy-
3,7-dimethyl
10.27 12.92 316.00
16. Benzophenone 12.54 0.24 182.00
17. Phenol, 4-(2-
aminoethyl)
13.05 0.18 137.00
18. 2,2-
methylenediphenol
14.21 0.57 200.23
19.
4,4'-
methylenediacetami
de
14.76 0.16 282.33
20. Tyramine 14.85 0.15 181.00
Total 100.00
From the result, the product (peak 15), lose a
common molecule of C=O (-28 Da) forming the
conjugation product with the m/z 316 [M+H]
+
ion.
Figure 5, also showed that the fragment of m/z 154
[M+H]
+
and m/z 181 [M+H]
+
were characteristic for
citronellal (C
10
H
18
O) and L-tyrosine (C
9
H
11
NO
3
)
respectively. However, these results need to be
further analysed to ensure that the product formed is
a true conjugate product (imine compound).
2. 00 4. 00 6.00 8. 00 10. 00 12.00 14. 00 16.00 18. 00 20.00 22. 00
2000000
4000000
6000000
8000000
1e+07
1. 2e+07
1. 4e+07
1. 6e+07
1. 8e+07
2e+07
2. 2e+07
2. 4e+07
2. 6e+07
2. 8e+07
Ti me-->
A
bundance
TI C: SAMPEL 2. D
3. 70
5.74
6. 31
6.49
6. 87
7. 03
7. 16
7.30
7. 50
7.73
7. 86
7.98
8.50
8. 64
10. 27
12.54
13. 05
14.22
14. 75 14.84
Conjugation Reaction between Citronellal and L-Tyrosine and Its Antimicrobial Properties against Bacteria and Fungi
77

Figure 5: The GC-MS analysis of fraction 2 isolated from
conjugation product (peak 15) at RT 10.27.
3.4 Antimicrobial Activity
The antifungal activity of the conjugation product
against Candida albicans is presented in Figure 6C.
The results showed that the conjugation product at
concentration of 10 and 50 mg/ml has no activity
against Candida albicans. While, the positive control
(nystatin) showed antifungal activity with the
diameter of inhibition zone was 10.70 nm (Table 2).
Figure 6: Antimicrobial activity of the conjugation product.
A. against S. aureus; B. E. coli; and C. C. albicans.
Table 2: The inhibition effect of the conjugation product
against Staphylococcus aureus, Escherichia coli, and
Candida albicans.
Sample
Diameter of inhibition
zone (mm)
S.
aureus
E.
coli
C.
albicans
C
-
Solvent nie nie nie
a
C
+
Nystatin nd
b
nd 10.70
Ciprofloxacin 21.78 nd nd
Gentamycin nd 29.60 nd
Citronellal 8.51 nie nie
Product 10 mg/ml nie nie nie
50 mg/ml nie nie nie
Note: C
-
: negative control; C
+
: positive control; nie: no
inhibition effect; nd: not determined.
The antibacterial activity of the conjugation
product also tested, and the results exhibited that the
product was has no antibacterial activities on
Staphylococcus aureus and Escherichia coli at
concentrations used. In this assay, we used
ciprofloxacin and gentamycin as positive controls for
Staphylococcus aureus and Escherichia coli
respectively (Table 2).
The results also showed that, ciprofloxacin,
gentamycin and citronellal (as precursor for synthesis
of conjugation product) have antibacterial activities
with the diameter inhibition zone were 21.78; 29.60;
and 8.51 mm respectively (Figure 6B and 6C). In the
conclusion, the antimicrobial assays showed that the
conjugation product have no inhibition effect against
Staphylococcus aureus, Escherichia coli, and
Candida albicans at concentration tested.
4 CONCLUSION
The conjugated product between citronellal and L-
tyrosine produces a powdery and yellowish-white
product, with percentage of yield was 71.12%. The
TLC analysis showed that a band with the Rf value of
0.85 was thought to be the conjugation product. The
GC-MS analysis showed that the fragment ion at m/z
316 [M+H]
+
, lose a common molecule of C=O (-28
Da), was expected to be conjugation product. The
antimicrobial assays showed that the conjugation
product at the concentrations of 10 and 50 mg/ml
have no inhibition effect on Staphylococcus aureus,
Escherichia coli, and Candida albicans.
ACKNOWLEDGEMENTS
The authors thank to Atsiri Research Center (ARC)
and Herbal Medicine Research Center (ProHerbal) of
Universitas Syiah Kuala for their support of this
study.
REFERENCES
Adhikari, S., Saha, S., Bandyopadhyay, T, K., Ghosh, P.,
2015. Efficiency of ISSR Marker for Characterization
of Cymbopogon Germplasms and Their Suitability in
Molecular Barcoding, Plant Systematics and Evolution,
301(1), 439–450.
Al-Garawi, Z, S., Tomi, I, H., Al-Daraji, A, H., 2012.
Synthesis and Characterization of New Amino Acid-
Schiff Bases and Studies Their Effects on the Activity
of ACP, PAP and NPA Enzymes (In Vitro), E-journal
Chem, 9(2), 962–969.
Anderson, J, A., Churchill, G, A., Autrique, J, E., Tanksley,
S, D., Sorrells, M, E., 1993. Optimizng Parental
Selection for Genetic Linkage Maps, Genome, 36(1),
181–186.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
78

Finland M, Nichols R 1957 Practitioner 179:84.
Griffith, R, C., Gentile, R, J., Davidson, T, A., Scott, F, L.,
1979. Convenient One-Step Synthesis of N-
Substituted. Alpha.-Methylphenethylamines Via
Aminomercuration-Demercuration, Jurnal of Organic
Chemistry, 44(20), 3580-3583.
Hong Y, Junkai L, Shengtao X, Zheying Z, Jinyi X 2017
Exp. Opin. Drug Disc. 12 121–140.
Lewis, K., Klibanov, A, M., 2005. Surpassing Nature:
Rational Design of Sterile-Surface Materials, Trends in
Biotechnology, 23(7), 343-348.
Martinez, D, M., Barcellos, A., Casaril, A, M., Perin, G.,
Schiesser, C, H., Callaghan, K, L., Lenardão, E, J.,
Savegnago, L., 2015. Twice Acting Antioxidants:
Synthesis and Antioxidant Properties of Selenium and
Sulfur Containing Zingerone Derivatives, Tetrahedron
Lett, 56, 2243–2246.
Murray, J, M., 2010. Fundamental of organic chemistry,
seventh edition; Cornell University. Brooks/Cole 20
Davis Drive: Belmont CA 94002-3098 USA.
Rukayadi, Y., Hwang, J, K., 2006. Effect of Coating the
Wells of A Polystyrene Microtiter Plate with
Xanthorrhizol on the Biofilm Formation of
Streptococcus Mutans, Journal of Basic Microbiology,
46(5), 410-415.
Rusdin F, Hardi Y, Syamsuddin 2018 Kovalen 1
Sangwan, N., Yadav, U., Sangwan, R., 2001. Molecular
Analysis of Genetic Diversity in Elite Indian Cultivars
of Essential Oil Trade Types of Aromatic Grasses
(Cymbopogon species), Plant Cell Reports, 20, 437–
444.
Silverstein, R, M., Webster, F, X., Kiemle, D, J., 2005.
Spectrometric identification of organic compounds,
New York, 7
th
eddition.
Tina Lütke-Eversloh 2007) Applied microbiology and
biotechnology 77(4) 10-31.
Ys H 2015 J. Nat. Sci. 4 111–118.
Ys, H., Rusdin, F., Syamsuddin, Rahim, E, A., 2019.
Comparison Analysis Between Experiment and
Computational Chemistry Data on Citronellal and
Tyrosine Conjugation, IOP Conf. Series: Journal of
Physics: Conf. Series, 1242.
Conjugation Reaction between Citronellal and L-Tyrosine and Its Antimicrobial Properties against Bacteria and Fungi
79
