
Simple Antimicrobial Labels from Cinnamon Oil Added to
Recycled Paper
Agustina Arianita Cahyaningtyas
1
, Retno Yunilawati
3
, Bunda Amalia
1
, Windri Handayani
2
and Cuk
Imawan
3
1
Badan Penelitian dan Pengembangan Industri, Kementerian Perindustrian
2
Departemen Biologi, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Indonesia,
Kampus Depok, Indonesia 16424
3
Departemen Fisika, Fakultas Matematika dan Ilmu Pengetahuan Alam, Universitas Indonesia,
Kampus Depok, Indonesia 16424
Keywords: Essential Oils, Cinnamon Oil, Antimicrobial Label, Recycled Paper.
Abstract: Essential oils are one of the antimicrobial agents that are safe for food, and thus can be used as an
antimicrobial label to extend the shelf life of food products. This study aims to prepare antimicrobial labels
and to investigate their activities in shrimp. Antimicrobial labels are made using cinnamon oil in the
recycled paper as a simple matrix. Cinnamon oil was tested on Gram-positive Staphylococcus aureus and
Gram-negative bacteria Escherichia coli using the paper disk diffusion method. From the results obtained,
cinnamon oil has both antimicrobial activities. Cinnamon oil is also characterized using Gas
Chromatography-Mass Spectrometry (GC-MS) to determine the level and presence of compounds suspected
of having antimicrobial activity. Cinnamon oil has interactions with recycled paper functional groups as
measured by Fourier Transform Infrared Spectroscopy (FTIR). Testing of antimicrobial labels on shrimp
shows that the Total Volatile Basic Nitrogen (TVB-N) value is better than without the label. From the
results of antimicrobial activity, can be seen that cinnamon oil applied to recycled paper has the potential to
be used as a simple antimicrobial label.
1 INTRODUCTION
Fresh shrimp is very easy to damage. Many methods
have been carried out to maintain the freshness and
shelf life of shrimp. The use of synthetic
preservatives to maintain the freshness and quality
of shrimp can endanger health. At present natural
preservatives with excellent antimicrobial properties
have been searched and implemented as safe
alternatives in seafood processing to extend shelf
life. Natural preservatives commonly used include
plant extracts, bacteriocins, bioactive peptides,
chitosan and chitooligosaccharide, and essential oils
(Olatunde and Benjakul, 2018). Essential oils from
aromatic plants have antimicrobial properties and
are safe to add to food or food packaging (Santos et
al., 2017).
Currently, some researchers are developing the
addition of essential oil as an antimicrobial to the
paper matrix. Researches on adding essential oil as
an antimicrobial and antifungal to paper matrix that
have been carried out are carvacrol (Mascheroni et
al., 2011) and cinnamon essential oil (Echegoyen
and Nerin, 2014). From the research that has been
done, the addition of essential oil to the paper matrix
mostly uses a coating method that requires
applicator coating equipment. Therefore, necessary
to develop a preparation method that is simple,
practical, can be used as an antimicrobial, and
integrated with product packaging.
This research aims to develop a simple
antimicrobial label by using cinnamon oil. As the
matrix of this simple antimicrobial label is recycled
paper. From studies on active paper packaging that
have been done, no one has ever used a matrix of
recycling paper. The use of recycled paper can
increase the added value of the recycled paper. Also,
the recycled paper easily absorbs essential oils
compared to other types of paper. The preparation
method of the label is simple, by dropping cinnamon
oil on the circular shape of the recycled paper. The
simple antimicrobial label is then tested to detect the
60
Arianita Cahyaningtyas, A., Yunilawati, R., Amalia, B., Handayani, W. and Imawan, C.
Simple Antimicrobial Labels from Cinnamon Oil Added to Recycled Paper.
DOI: 10.5220/0009956300600066
In Proceedings of the 2nd International Conference of Essential Oils (ICEO 2019), pages 60-66
ISBN: 978-989-758-456-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

characterization, antimicrobial activity, and TVB-N
of the shrimp after applied by a label.
2 MATERIALS AND METHOD
2.1 Materials
The simple antimicrobial label was made using
materials recycle paper purchased from local
stationery shop and cinnamon oil purchased from
Nusa Aroma local essential oils company in
Indonesia.
2.2 Antimicrobial Label Preparation
The simple antimicrobial labels were composed of
the cinnamon oil and the matrix made from recycled
paper. The labels were prepared by dropping of
50 µL cinnamon oil on circular shape cutting of the
recycle paper with a diameter of 6 mm. The label
then dried in room temperature for 5 minutes ready
for use.
2.3 Characterization
2.3.1 Cinnamon Oil Characterization using
GCMS
Cinnamon oil was characterized using a mass
spectrometer detector (GCMS), to find out the
chemical compounds contained in cinnamon oil. The
tools used are GC / MS with Agilent 6890 series
specifications with capillary column HP-5MS, 30
m x 0.25 mm id x 0.25 µm film thickness. As the
carrier gas was used helium gas at constant pressure.
The essential oil was injected with a volume of 1 µL
(split ratio of 25: 1). The oven temperature was
programmed from 60 °C - 240 °C for an increase of
3 °C per minute until reaching 250 °C.
2.3.2 Characterization using FTIR
FTIR characterization is used to monitor label
activity. Tests are carried out on blank paper and
labels before and after storing shrimp and carried out
every day. The blank and the label were measured
on the Seri Nicolet iS5 FTIR spectrometer. All
spectra were taken in the spectral range of 4000 cm
-1
until 500 cm
-1
.
2.4 Antimicrobial Activity Assay
2.4.1 Direct Contact Agar Diffusion Tests
Direct contact agar diffusion tests determined by the
paper disk diffusion method using strain type of
Staphylococcus aureus NBRC 100910 and
Escherichia coli NBRC 3301 in The Mueller Hinton
Agar. 10 mL of molten media was put into a sterile
petri dish (d = 90 mm) until it became solid for
5 minutes. 10 µL bacterial culture 10
-6
CFU / mL is
added with 10 mL medium into the tube and mixed
slowly with inoculating before pouring on the top
surface of the molten media and allowed to dry for 5
minutes. The negative control (sterile distilled
water), positive control (tetracycline 15 µg / mL),
and cinnamon oil with a concentration of 1000 µg /
mL are poured on 6 mm discs, where the volume for
each disc is 10 µL. The disc is then placed on the
surface of the medium then incubated at 35 °C for
18 hours. After completion of incubation, a clear
zone is formed around the disc measured.
2.4.2 Vapour Phase Agar Diffusion Tests
This vapour phase agar diffusion uses the method
used by (Wang et al., 2016). The vapour phase agar
diffusion test technically has the same method as the
direct contact method. The test uses a 6 cm diameter
petri dish, bacterial culture, filter disc size, and
cinnamon oil adding. The disk filter is placed in the
middle of the lid of the petri dish. The dishes are
then sealed using a paraffin laboratory to prevent
evaporation of the test compound. Incubation ran at
32 °C for 24 hours. The clear zone diameter was
measured.
2.5 Total Volatile Basic Nitrogen
(TVB-N)
The shrimp used for this experiment were fresh
obtained from the local market. Shrimp after being
bought directly delivered to the laboratory and
prepared as soon as possible for observation. The
shrimp weighed as much as 10 g and then put into a
PVC square packaging. Then a simple antimicrobial
label was attached to the top of PVC square
packaging containing the shrimp, placed indirectly
in contact with the shrimp. The distance between the
label and shrimp is about 1 cm. The PVC square
packaging is then tightly closed. Observation of
shrimp freshness was carried out at room
temperature for three days. During this time, TVB-N
levels were measured every day, as a control used
Simple Antimicrobial Labels from Cinnamon Oil Added to Recycled Paper
61
shrimp that are packaged without using simple
antimicrobial label. Measurement of TVB-N levels
in shrimp according to the Total Volatile Basic
Nitrogen (TVB-N) method based on Commission
Regulation (EC) No 2074/2005 (EC, 2005).
3 RESULT AND DISCUSSION
3.1 Chemical Compounds of the
Cinnamon Oil
The method used to analyse volatile oils for many
years is using gas chromatography. GC-MS is the
most appropriate technique used to identify the
compounds contained in essential oils. The
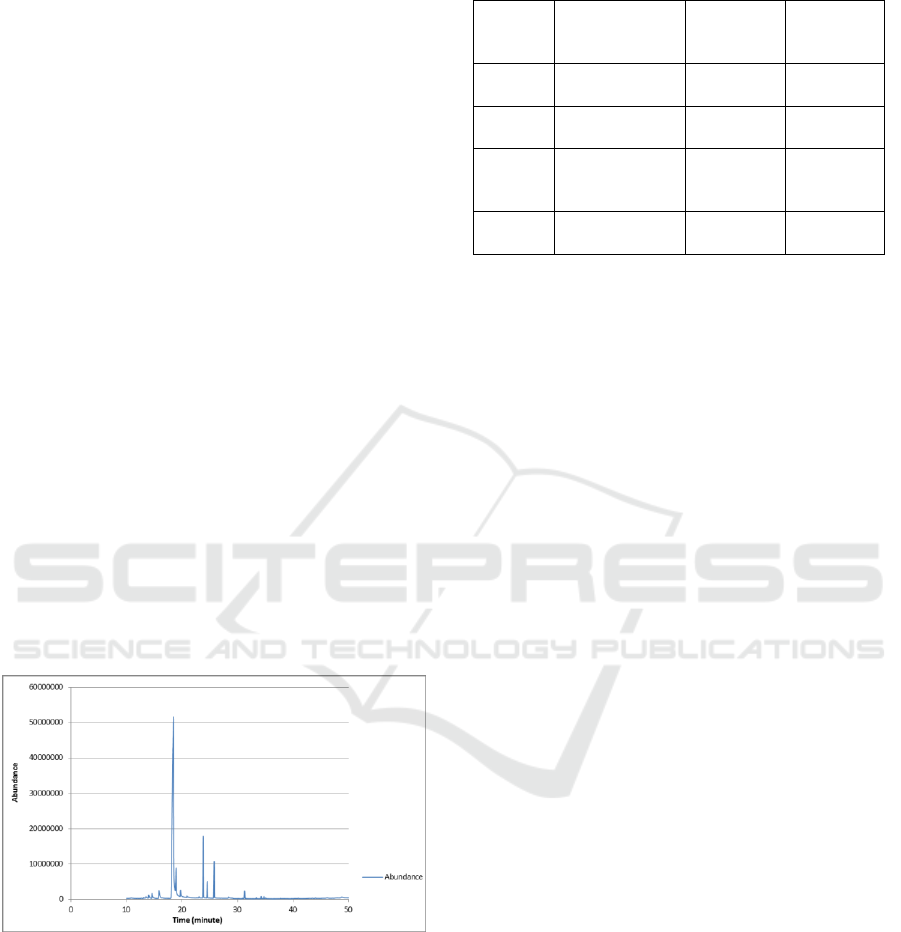
chromatogram profile of cinnamon oil is shown in
Figure 1, and the results of the characterization of
the chemical compounds listed in cinnamon are
shown in Table 1.
A total of four different components, with
different retention times, were indicated by the
chromatogram in Figure 1. Based on Table 1, GC-
MS analysis revealed that different chemical
compositions were identified in cinnamon oils,
including cinnamaldehyde, iso-bornyl acetate,
cinnamaldehyde dimethyl acetyl, and cynamil
alcohol. The main component of cinnamon oil is
cinnamaldehyde (83.87%). This result is the same as
some of the results of previous studies conducted by
Figure 1: GCMS chromatogram of the cinnamon oil.
Table 1: Chemical component identified of cinnamon oil
with GCMS.
Retenti
on time
Identified
compound
Molecular
formula
Relative
percentage
area (%)
18.501
Cinnamaldehy
de
C
9
H
8
O
83.87
18.913
Iso-bornyl
acetate
C
12
H
20
O
2
4.71
23.817
Cinnamaldehy
de dimethyl
acetal
C
11
H
14
O
2
7.31
25.788
Cynamil
alcohol
C
11
H
12
O
2
4.10
researchers that cinnamaldehyde is a major
component of cinnamon oil (Gotmare and Tambe,
2019; Dwijatmoko, 2016; Li, Kong and Wu, 2013).
Cinnamaldehyde is a compound containing aldehyde
groups and conjugated double bonds outside the ring
(Sachdeva et al., 2017). Cinnamaldehyde is an
organic mixture that gives wood a sweet taste and
smell (also known as cinnamic aldehyde). This
organic compound is significant to inhibit bacterial
growth (Ashakirin et al., 2017). Antimicrobial
activity of cinnamaldehyde was found against E. coli
and staphylococcus aureus. Cinnamaldehyde plays a
role in disrupting bacterial cell membranes (Firmino
et al., 2018).
3.2 Antimicrobial Activity of
Cinnamon Oil
The antimicrobial activity of cinnamon oil was
analysed for gram-positive bacteria (S. aureus) and
gram-negative bacteria (E. coli). The results of the
analysis of antimicrobial activity are shown in
Figure 2 and Table 2.
Antimicrobial ability is shown from the diameter
of the inhibition zone (measured the clear area) as
shown in Figure 2. Based on Table 2, cinnamon oil
has antimicrobial activity against Escherichia coli
and Staphylococcus aureus. In the paper disc
diffusion method, the area of inhibition depends on
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
62
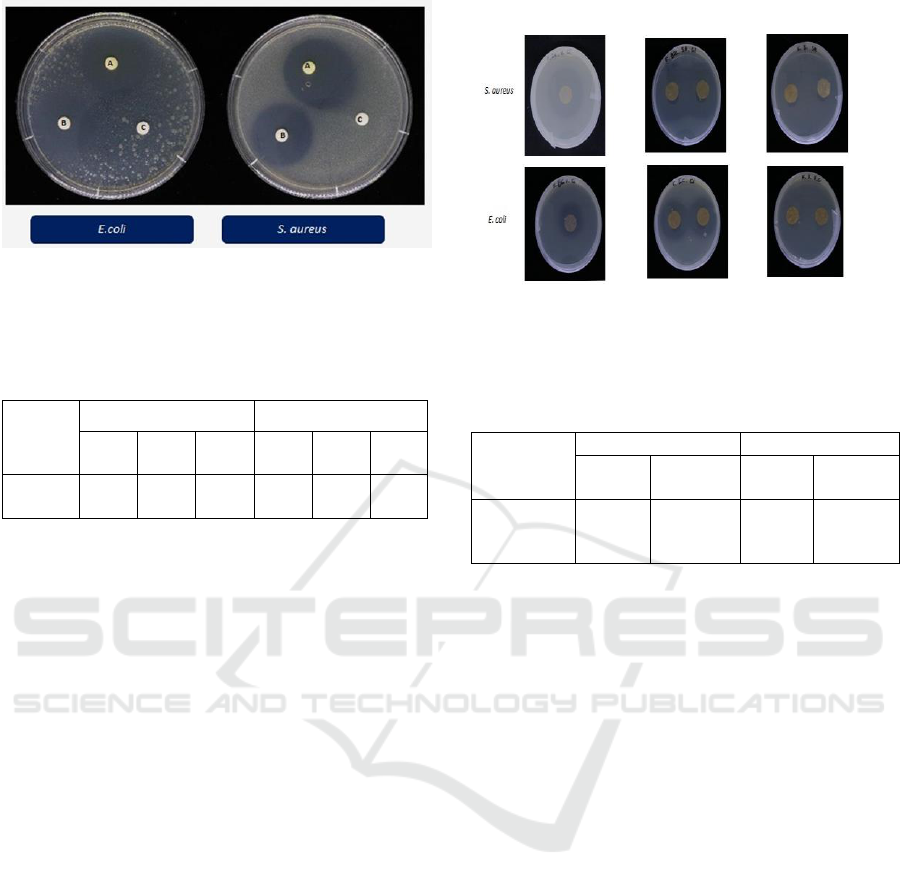
Figure 2: The inhibition zone cinnamon oil by the paper
disc diffusion method (a : positive control, tetracycline; b :
sample, cinnamon oil; c : negative control (sterile distilled
water)).
Table 2: Antimicrobial activity of cinnamon oil.
Essential
Oil
E. coli (mm)
S.aureus (mm)
Sample
Control
(-)
Control
(+)
Sample
Control
(-)
Control
(+)
Cinnamon
oil
34
0
30
35
0
38
the ability of the essential oil to diffuse evenly to
medium and also releases volatile compounds from
essential oil. Inhibition zone of cinnamon oil against
E. coli is 34 mm, and against a S. aureus is 35 mm.
These results are similar with previous research
conducted by (Adinew, 2014) reported that
cinnamon oil shows an inhibitory effect against the
gram-positive bacteria (Bacillus cereus,
Micrococcus luteus, Staphylococcus aureus, and
Enterococcus faecalis) and gram-negative bacteria
(Alcaligenes faecalis, Enterobacter cloacae, and
Escherichia coli).
3.3 Antimicrobial Activity of the
Simple Antimicrobial Label
Cinnamon oil that has been added to the recycle
paper is then analysed for its antimicrobial activity
compared to the blank, to determine the
antimicrobial ability of the label. Analysis of
antimicrobial activity on labels is done by paper disk
(direct contact) and vapour phase diffusion test
because when applied to shrimp analysed using the
phase diffusion vapour method. The analysis results
are shown in Figure 3 and Table 3.
Based on Figure 3, the blank (only recycled
paper) does not show the inhibition zone. The
absence of the inhibition zone indicates that recycle
paper does not have the antimicrobial ability.
Figure 3: The inhibition zone simple antimicrobial label
by the paper disc and vapour phase agar diffusion method.
Table 3: Antimicrobial activity of simple antimicrobial
label.
Direct contact
Vapor
E. coli
(mm)
S.aureus
(mm)
E. coli
(mm)
S.aureus
(mm)
Recycle
Paper
cinnamon oil
36.98
50.31
28.75
44.54
Meanwhile, after adding cinnamon oil to the
recycle paper, the inhibition zone was seen, which
stated that the label has the antimicrobial ability. The
antimicrobial label has an antimicrobial ability
against the gram-positive bacteria (Staphylococcus
aureus) and gram-negative bacteria (Escherichia
coli). It can be seen from Table 3 that a clear zone or
diameter of the inhibition zone of 36.98 cm (E. coli)
and 50.31 cm (S. aureus) for the direct contact
method, while the vapour diffusion method is 28.75
cm (E. coli) and 44.54 cm (S. aureus). Antimicrobial
labels have a better antimicrobial ability against S.
aureus than E. coli, both from the test results using
vapour or direct contact. This result is the same as
the results of research conducted by (Zhang et al.,
2016), which states that E. coli is more resistant to
cinnamon oil than S. aureus. This phenomenon
probably due to differences in the structure of the
bacterial outer membrane. E. coli has a thick layer of
lipopolysaccharide on its outer membrane that
covers the cell wall, whereas S. aureus has only a
single peptidoglycan layer structure. Therefore E.
coli is more resistant to essential oils (hydrophobic
substance) compared with S. aureus.
RP-CO : Vapor
RP-CO : Direct contact
Blank : Direct contact
Simple Antimicrobial Labels from Cinnamon Oil Added to Recycled Paper
63
3.4 Total Volatile Basic Nitrogen
(TVB-N)
The enzymatic and bacteriological activity can
quickly reduce the protein content and quality of
stale seafood, some ammonia, trimethylamine,
dimethylamine, and other volatile basic nitrogen
compounds are produced, which together are called
TVB-N (Fallah et al., 2016). Total volatile basic
nitrogen (TVB-N) is one method that is often used to
measure seafood quality and, most commonly, as an
indicator of chemical decay in marine products
(Altissimi et al., 2017). TVB-N analysis was
performed to find out the freshness of shrimp stored
without or using simple antimicrobial labels. TVB-N
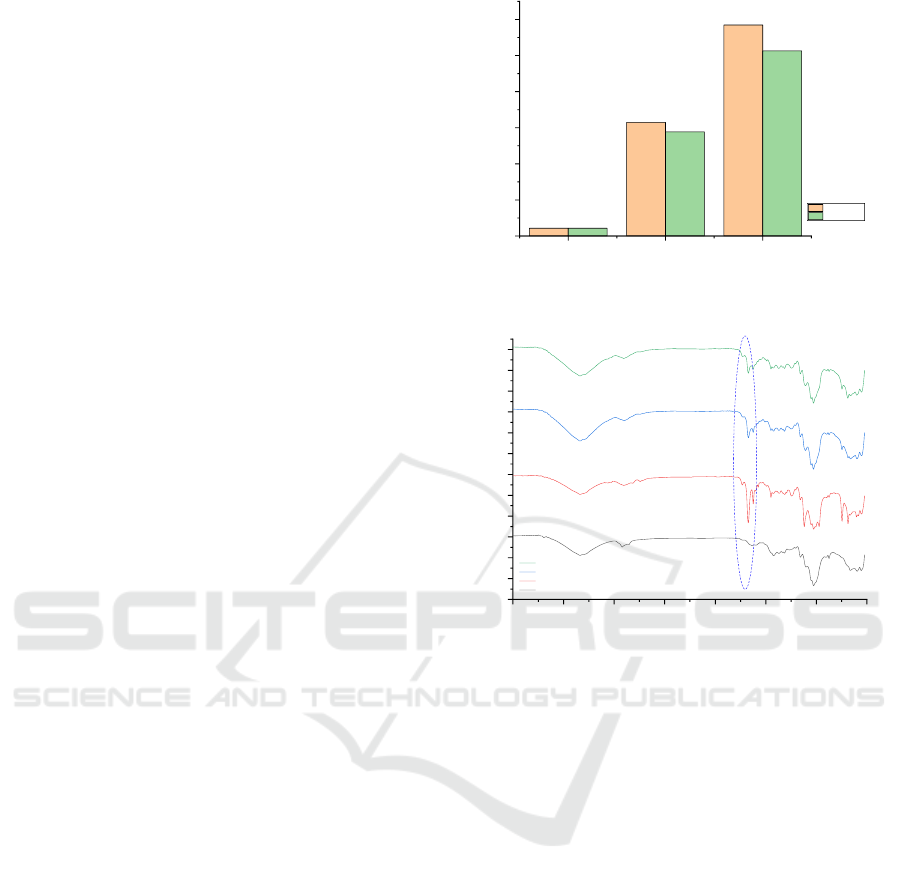
analysis results are shown in Figure 4.
Based on the graph in Figure 4 shows that the
value of TVB-N is increasing. It is consistent with
the results of previous research conducted by
(Chakrabortty et al., 2017), which states that the
value of TVB-N increases with storage time. The
low value of TVB-N is an indication of the quality
of fresh shrimp, while the high value of TVB-N may
be due to the action of the enzyme autolysis and
spoilage bacteria. TVB-N values for “high quality”
quality up to 25 mg / 100 g, “good quality” up to
30 mg / 100 g, “limit of acceptability” up to
35 mg / 100 g, and “spoiled” above 35 mg / 100 g
(Jinadasa, 2014). From the graph in Figure 4 also
shows that the value of TVB-N for shrimp stored
using simple antimicrobial labels is lower than
shrimp stored without using simple antimicrobial
labels. It shows that simple antimicrobial labels can
be used to maintain the freshness of shrimps,
however further research is needed to determine the
optimization of the addition of cinnamon oil to
recycled paper.
3.5 Fourier Transform Infrared
Spectroscopy
The functional group of the label was analysed using
FTIR for three days to determine changes in
functional groups that occur during that day. Figure
5 displays the spectra of the simple antimicrobial
labels.
Figure 4: Total volatile base nitrogen (TVB-N) of shrimp.
Figure 5: FTIR spectra of recycling paper and simple
antimicrobial label.
Based on Figure 5, the IR characteristic
fingerprint for cinnamon oil is mostly in the range of
1800 cm
-1
- 600 cm
-1
(Li et al., 2013). In the IR
spectra, it is shown that the absorbance band at 1690
cm
-1
- 1760 cm
-1
revealed the presence of C = O
bond for aldehyde from cinnamaldehyde (Adinew,
2014). These results are consistent with the results of
the analysis using GCMS, which shows that the
main component of cinnamon oil is cinnamaldehyde.
The IR spectroscopy spectrum display characteristic
bands corresponding to aromatic CH bonds
(between 3000 cm
-1
and 3100 cm
-1
), CH alquenes
(between 3020 cm
-1
and 3080 cm
-1
), and C = C
(between 1640 cm
-1
- 1680 cm
-1
) (Singh et al.,
2011). From the Figure, the C = O intensity of
cinnamaldehyde is decreasing. It is because
cinnamaldehyde has to be released from the label.
0 1 2
0
20
40
60
80
100
120
Total volatile base nitrogen (mg/100 g)
Days
Without label
With label
4000 3500 3000 2500 2000 1500 1000 500
Transmittance
Wavenumber (1/cm)
RP-Cinnamon day 2
RP-Cinnamon day 1
RP-Cinnamon day 0
RP
C=O aldehyde
(1690-1760 cm
-1
)
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
64

4 CONCLUSION
In this experiment, cinnamon oil has antimicrobial
ability against the gram-positive bacteria
(Staphylococcus aureus) and gram-negative bacteria
(Escherichia coli). Simple antimicrobial labels from
cinnamon oil added to the recycled paper also have
antimicrobial ability against the gram-positive
bacteria (Staphylococcus aureus) and gram-negative
bacteria (Escherichia coli). The results obtained
from this experiment indicated that this simple
antimicrobial label could be used to maintain the
freshness of shrimps. Further research is needed to
determine the optimization of the addition of
cinnamon oil to recycled paper.
ACKNOWLEDGEMENT
This research supported by PSNI (Penelitian
Strategis Nasional Institusi) from Kementerian
Riset, Teknologi, dan Perguruan Tinggi Republik
Indonesia No NKB-
1798/UN2.R3.1/HKP.05.00/2019. We also thank the
Center of Excellence Biology Resources Genome
Study (CoE IBR-GS) FMIPA UI and the Center for
Chemical and Packaging (CCP) for the facilities and
equipment to support this research.
REFERENCES
Adinew, B., 2014. GC-MS and FT-IR Analysis of
Constituents of Essential Oil From Cinnamon Bark
Growing in South-west of Ethiopia, International
Journal of Herbal Medicine, 1(6), 22–31.
Altissimi, S., Mercuri, M. L., Framboas, M., Tommasino,
M., Pelli, S., Benedetti, F., Di Bella, S., Haouet, N.,
2017. Indicators of Protein Spoilage in Fresh and
Defrosted Crustaceans and Cephalopods Stored in
Domestic Condition, Italian Journal of Food Safety,
6(4), 217–221.
Ashakirin, S. N., Tripathy, M., Patil, U, K., Majeed, A, B,
A., 2017. Chemistry and Bioactivity of
Cinnamaldehyde : A Natural Molecule of Medicinal
Importance, International Journal of Pharmaceutical
Sciences and Research, 8(6), 2333–2340.
Chakrabortty, A., Aziz, M, A., Rashad, M, A., Quality, F.,
2017. Study on Protein Losses and Total Volatile Base
Nitrogen (TVBN) of Fresh Water Prawn (Galda) in
the Shrimp Value Chain of Bagerhat Region,
Bangladesh for the Development of Sea Food Quality,
Journal of Engineering Science, 8(1), 79–82.
Dwijatmoko, M, I., 2016. Effect of Cinnamon Essential
Oils Addition in the Sensory Attributes of Dark
Chocolate, Nusantara Bioscience, 8(2), 301–305.
EC., 2005. Commission regulation (EC) No 2074/2005,
Official Journal of the European Union.
Echegoyen, Y., Nerin, C., 2014. Performance of An
Active Paper Based on Cinnamon Essential Oil in
Mushrooms Quality, Food Chemistry, 1–28.
Fallah, F., Ebrahimnezhad, Y., Maheri-Sis, N., Ghasemi-
Sadabadi, M., 2016, The Effect of Different Levels of
Diet Total Volatile Nitrogen on Performance, Carcass
Characteristics and Meat Total Volatile Nitrogen in
Broiler Chickens, Archives Animal Breeding, 59(2),
191–199.
Firmino, D, F., Cavalcante, T, T, A., Gomes, G, A.,
Firmino, N, C, S., Rosa, L, D., De Carvalho, M, G.,
Catunda, F, E, A., 2018. Antibacterial and Antibiofilm
Activities of Cinnamomum Sp. Essential Oil and
Cinnamaldehyde: Antimicrobial Activities, The
Scientific World Journal, 2018, 1–9.
Gotmare, B., Tambe, E., 2019. Identification of Chemical
Constituents of Cinnamon Bark Oil by GCMS and
Comparative Study Garnered from Five Different
Countries, 19(1).
Jinadasa, B, K, K, K., 2014. Determination of Quality of
Marine Fishes Based on Total Volatile Base Nitrogen
Test (TVB-N), Nature and Science, 12(5), 106–111.
Li, Y, Q., Kong, D, X., Wu, H., 2013. Analysis and
Evaluation of Essential Oil Components of Cinnamon
Barks using GC-MS and FTIR Spectroscopy,
Industrial Crops and Products, 41(1), 269–278.
Mascheroni, E., Guillard, V., Gastaldi, E., Gontard, N.,
Chalier, P., 2011. Anti-Microbial Effectiveness of
Relative Humidity-Controlled Carvacrol Release from
Wheat Gluten/Montmorillonite Coated Papers, Food
Control, 22(10), 1582–1591.
Olatunde, O, O., Benjakul, S., 2018. Natural Preservatives
for Extending the Shelf-Life of Seafood: A Revisit,
Comprehensive Reviews in Food Science and Food
Safety, 17(6), 1595–1612.
Sachdeva, A., Vashist, S., Chopra, R., Puri, D., 2017.
Antimicrobial Activity of Active Packaging Film to
Prevent Bread Spoilage, International Journal of Food
Science and Nutrition, 2(4), 29–37.
Santos, R, R., Andrade, M., Melo, N, R., Sanches-Silva,
A., 2017. Use of Essential Oils in Active Food
Packaging: Recent Advances and Future Trends,
Trends in Food Science and Technology, 61, 132–140.
Singh, P., Andola, H, C., Rawat, M, S, M., Pant, G, J, N,
P., Purohit, V K., 2011. Fourier Transform Infrared
(FT-IR) Spectroscopy in An-Overview, Research
Journal of Medicinal Plant, 5(2), 127–135.
Simple Antimicrobial Labels from Cinnamon Oil Added to Recycled Paper
65

Wang, T, H., Hsia, S, M., Wu, C, H., Ko, S, Y., Chen, M,
Y., Shih, Y, H., Shieh, T, M., Chuang, L, C., Wu, C,
Y., 2016. Evaluation of the Antibacterial Potential of
Liquid and Vapor Phase Phenolic Essential Oil
Compounds Against Oral Microorganisms, Plos One,
11(9), 1–17.
Zhang, Y., Liu, X., Wang, Y., Jiang, P., Quek, S, Y., 2016.
Antibacterial Activity and Mechanism of Cinnamon
Essential Oil Against Escherichia Coli and
Staphylococcus aureus, Food Control, 59, 282–289.
ICEO 2019 - 2nd International Conference of Essential Oil Indonesia
66
