
Design Business Process Management Model for Pharmaceutical with
Good Manufacture Practice and Good Distribution Practice in Indonesia
Erick Fernando
1
, Meyliana
1
and Surjandy
1
1
Information Systems Department, School of Information Systems, Bina Nusantara University, Jakarta, Indonesia 11480
Keywords:
Design model, Business Process Management, Pharmaceutical, Good Manufacture Practice, Good Distribu-
tion Practice
Abstract:
This study aims to present the business processes management of the pharmaceutical industry combined with
supply chain management processes Good Drug Distribution (GDP) and Good Manufacturing Practice (GMP).
Thereby can complete business process management (BPM) by government regulations, giving a good picture
of business processes within the company or pharmaceutical industry by GDP and GMP. This research was
conducted with a qualitative approach, which was carried out by reviewing the literature of several literary
articles related to the subject of this research and conducted a discussion forum with the pharmaceutical in-
dustry in Indonesia. Business process management model with a combination of GMP and GDP approaches
to improve efficiency and effectiveness by creating automation, speed, and process accuracy in change man-
agement. With this BPM model, the BPM can improve business processes and supply chain management
processes in the pharmaceutical industry, and the BPM can provide efficiency and effectiveness in the in-
dustrial drug distribution process. New BPM can reduce the problems in the pharmaceutical industry in the
circulation of medicines and the availability of medicines in the market.
1 INTRODUCTION
The development of information technology in the
current era provides a change in competitiveness that
is so terrible. The effect of this change has an im-
pact on companies today, so companies try to always
improve their competitiveness by changing all orien-
tations from functional to process (Buttigieg et al.,
2016) (Smolnik et al., 2011) (Ramos-Merino et al.,
2019)(Rentes et al., 2019) (Venkatraman and Venka-
traman, 2019). This change leads to the concept
of business process management (BPM) by direct-
ing company changes in the systematic identification,
modelling, and process improvement of company per-
formance. In improving this process, a process of
planning and implementation must lead to improve-
ment and is carried out by combining knowledge in
analyzing the strengths and weaknesses of the com-
pany and its resources to suit the company (Couckuyt
and Van Looy, 2019) (Fernando et al., 2018) (Kitsios
and Kamariotou, 2019) (Tsakalidis et al., 2019).
BPM has a scope and objectives related to changes
and improvements that stem from the analysis of
problems that occur in companies that hurt the ef-
fectiveness and efficiency of the process that is be-
ing implemented (Bitkowska et al., 2018) (Malinova
and Mendling, 2018) (Niehaves et al., 2014) . The
main process in supporting the company’s develop-
ment process that is increasing in the process, build-
ing a knowledge base that leads to all aspects of BPM
implementation (Brocke, 2018) (John, 2008). BPM
has been widely used in various companies with var-
ious fields, specifically in the pharmaceutical indus-
try. The pharmaceutical industry is an industry that
provides drugs on the market. From the focus group
discussion process, there are some problems in the in-
dustry that are most crucial in the manufacturing stage
is not being able to do good planning in fulfilling the
supply of drugs from the ingredients of making the
drug formula to packing material (Hamilton, 2013)
(Marcketti, 2005) (Puri and Ranjan, 2012). This pro-
cess is triggered by the uncertain supply of drug ingre-
dients and the unavailability of drugs at distributors
or influenced by ignorance of the number of drugs on
the market (Puri and Ranjan, 2012). Also, problems
arise in the distribution process that has problems with
many distributions that do not comply with the provi-
sions that lead to the circulation of illegal drugs in the
market. With this problem, the pharmaceutical indus-
try needs BPM for managing business processes that
178
Fernando, E., Meyliana, . and Surjandy, .
Design Business Process Management Model for Pharmaceutical with Good Manufacture Practice and Good Distribution Practice in Indonesia.
DOI: 10.5220/0009907501780183
In Proceedings of the International Conferences on Information System and Technology (CONRIST 2019), pages 178-183
ISBN: 978-989-758-453-4
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

are carried out following its supply chain (material
providers, manufacturing industries, distributors, re-
tailers, consumers) so that they can be more effective
and efficient in the process. BPM can be combined
with GMP and GDP by government regulations.
This study aims to present or describe the manage-
ment of business processes of the pharmaceutical in-
dustry combined with supply chain management pro-
cesses, GMP, and GDP methods. Thereby can com-
plete business process management by government
regulations, giving a good picture of business pro-
cesses within the company or pharmaceutical indus-
try by good drug distribution and good manufactur-
ing practice. The development of BPM in this arti-
cle was developed an update to reach the final stage
starting from the supply of raw materials, the manu-
facturing process to the good distribution process for
consumers. This gets certainty from the outstanding
and quality drugs.
2 THEORY FOUNDATION
2.1 Supply Chain Management in the
Pharmaceutical Industry
The supply chain management (SCM) process in
the pharmaceutical industry is an activity carried out
from the process of raw materials being managed
into drugs to the distribution process to users and
guaranteeing or ensuring that drugs can be well re-
ceived, quality and in accordance with needs(Shao
and Ji, 2006) (Law, 2016) (Rovers and Mages, 2017).
In this SCM, the pharmaceutical supply chain com-
plexity results from the involvement of many large,
highly diverse independent organizations (Dachyar
and Novita, 2016) (Van der Aalst, 2013). The main
stakeholders in this supply chain include government
agencies, hospitals, clinics, drug manufacturers, drug
distributors, pharmaceutical chains, retailers (Indone-
sia, 2009). The same supply chain is responsible for
the distribution of existing drugs such as prescrip-
tion drugs, over-thecounter drugs, generic drugs, hard
drugs and drugs with narcotics that have different op-
erational objectives (Gr
¨
une et al., 2014) (Nwabueze,
2012).
space
Figure 1: Supply Chain Management (Tipton, 2015)
2.2 Business Process Management
Business process management is a model approach to
improve efficiency and effectiveness by building an
automation process, speed, and precision in the man-
agement of a change. This business process manage-
ment analyses existing performance processes then
makes changes by designing new processes by opti-
mizing existing or available processes using various
facilities and methodologies (John, 2008) (Ramos-
Merino et al., 2019). BPM is a dynamic system,
so you can always change what is needed continu-
ously according to the situation and conditions (John,
2008). BPM examines each increase in the company’s
performance to achieve business processes that are
managed with a maximum or optimum. The most im-
portant part of BPM is the process because of how it
can understand, manage and develop an organization
to provide products or services of greater or greater
value to partners or customers (Brocke, 2018)(Mali-
nova and Mendling, 2018). BPM can be supported by
technology to accelerate the processes carried out in
it. Also, many parties discuss GMP from a technolog-
ical or multi-person point of view (John, 2008).
In many companies today, many are implement-
ing BPM with the main reason for the company to
become more competitive in today’s fierce commer-
cial competition and its future (Niehaves et al., 2014)
(Van der Aalst, 2013). This competitive is because
the company has the challenge of being able to sur-
vive or be superior in business competition. Improve-
ments can be made by providing added value, increas-
ing productivity, reducing costs, and improving the
company’s business processes. These are some of the
benefits of BPM that will help a company navigate
business competition, namely: Improve business ca-
pacity, reduce costs and increase profits, increase effi-
ciency, improve visibilit(John, 2008) (Koster, 2009).
BPM main components Each Business Process
Management (BPM) solution has four main compo-
nents (Smolnik et al., 2011) (Van der Aalst, 2013):
2.2.1 Modelling
Users can define and design the structure of each busi-
ness process graphically.
Design Business Process Management Model for Pharmaceutical with Good Manufacture Practice and Good Distribution Practice in
Indonesia
179

2.3 Integration
In BPM, each element can be related in carrying out
the process so that collaboration or exchange of infor-
mation occurs to complete the goal.
2.3.1 Supervision
Users can monitor and control the performance of the
ongoing business processes and the performance of
each staff involved in the business process.
2.3.2 Optimization
Users can analyze and monitor a business process and
also take measures to improve their efficiency.
An excellent way to do this is to describe the pro-
cess view of the organization and the list of processes
from beginning to end, giving a more detailed view of
the process and the list of processes from beginning
to end of the organization.
In the design process, can group the processes that
occur, the grouping can be done on three levels:
1. Strategic processes: this level represents a strate-
gic process, which must ensure that the underly-
ing process meets and continues to meet the spec-
ified objectives.
2. Core processes: this level represents the principal
or main business activities of the organization
3. Support processes: this level describes noncentral
processes, which support the central operations of
the organization
2.4 BPM IN SCM PHARMACY
The pharmaceutical industry has continued to develop
due to globalization, consolidation, and regulatory
compliance. Manufacturing needs to combine more
efficient and quality-focused processes. The phar-
maceutical supply chain must be scalable and ag-
ile enough to adapt to changing scenarios and part-
ners worldwide. Research and development (R&D)
are under pressure to reduce costs and cycle times
(Koster, 2009) (Nwabueze, 2012) . When managed
care organizations limit medication forms and pro-
mote generic drugs, pharmaceutical companies de-
velop strategies to compete with drugs. Staying com-
petitive in this developing environment has never been
more challenging. Sales and marketing must over-
come regulatory demands by reassessing how they
manage more and more channels and partners (Hein
et al., 2015). Amid this background, efficient man-
agement of business processes is essential for sustain-
ability and growth. The standard BPM solution al-
lows organizations to dynamically manage workflows
and electronic form processing with a steady BPM.
2.5 Good Distribution Practice in
Indonesia
This guide is used as a reference to carry out the pro-
cess of distribution of medicines by interested par-
ties (pharmaceutical industry, pharmaceutical indus-
try, and community services (pharmacies, hospitals,
public health centres) at the hands of consumers. HK
03.1 .34.11.12.7542 of 2012 (BPOM RI, 2012) in-
cludes:
2.5.1 Quality Management
The quality system developed must be fully docu-
mented, and its effectiveness monitored. Within the
system, there must be a change control system that
includes the principles of quality risk management.
2.5.2 The Organization, Management, and
Personnel
The correct implementation and management of a
quality management system and the correct distri-
bution of the medications and/or ingredients of the
medications depend very much on the personnel who
carry them out.
2.5.3 Buildings and Equipment
Buildings and equipment to guarantee the protec-
tion and distribution of drugs and/or drug ingredients.
Buildings must be designed and adapted to ensure that
good storage conditions can be maintained, have ad-
equate security and sufficient capacity to allow safe
storage and handling of drugs, and the storage area is
equipped with adequate lighting to allow that all ac-
tivities are carried out accurately and safely.
2.5.4 Operational
All actions taken by the distribution facilities must en-
sure that the identity of the drugs and/or the ingredi-
ents of the drugs is not lost and that the specifications
listed on the packaging manage their distribution.
2.5.5 Self-inspection
Self-inspection should be carried out to monitor the
implementation and compliance with CDOB compli-
ance and to follow up on the necessary corrective
measures.
CONRIST 2019 - International Conferences on Information System and Technology
180

2.5.6 Complaints of Drugs and/or Drug
Ingredients That Are Returned, Allegedly
False, and Withdrawn
All complaints and other information about poten-
tially damaged medications should be collected, re-
viewed, and investigated by written procedures. The
responsible personnel must approve medications to be
resold by their authority.
2.5.7 Transportation
The transport process must apply appropriate trans-
port methods. The medicine must be transported with
storage conditions according to the information on the
package. Appropriate methods of transport should be
used, including transport by land, sea, air or a combi-
nation of the above.
2.5.8 Facilities based on Contract
All contract activities must be in writing between the
contract grantor and the contract recipient, and each
activity must comply with CDOB requirements.
2.5.9 Documentation
Good documentation is an essential part of the quality
management system. Written documentation must be
clear to avoid errors in verbal communication and to
facilitate follow-up, including batch history, instruc-
tions, and procedures.
Figure 2: Good Distribution Practice
Business processes carried out among them are:
1. Receipt of orders
This activity is to receive goods by carrying out
activities such as a collection of order letters,
write of order letters, correction of customer or-
ders.
2. Procurement of Goods
The process of procurement of goods carried out,
such as recapitulation of demand, determine the
procurement policy, procurement of goods, re-
lease of supplies, order goods to the main external
actors.
3. Manufacture invoice
This process will be carried out by verifying the
order letter, the approval of the transaction, the
printing of the invoice, the calculation of VAT, the
claim process, and the customer discount.
4. Delivery of goods
Some of the processes carried out include the de-
livery orders (DO) for customers, the preparation
of products, the shipment of products.
2.6 Good Manufacturing Practice
Good Manufacturing Practices in Figure 3 are : a
model used in the pharmaceutical industry at each
stage of processing from raw material control, for-
mula manufacturing validation, equipment validation,
manufacturing process validation, test method vali-
dation , audit and review functions, material testing,
testing of finished products, material control, han-
dling of raw materials, storage, packaging processes,
environmental monitoring, approval of manufacturing
instructions, approval of the use of materials, includ-
ing the use of water, exhaustive documentation to cre-
ate system quality (Van der Aalst, 2013). Good Man-
ufacturing practice consists of:
1. Trained Personal
2. Quality System with Traceable Documentation
and Records
3. Approved Materials (including Water)
4. Approved Manufacturing instructions
5. Controlled Materials Handling, Storage, Segrega-
tion, Packaging’s, and Labelling
6. Materials, intermediates and finished products
testing
7. Internal Audits and reviews
8. Validated test methods
9. Validated manufacturing processes
10. Validated Equipment’s
11. Approved Manufacturing Facilities
Design Business Process Management Model for Pharmaceutical with Good Manufacture Practice and Good Distribution Practice in
Indonesia
181
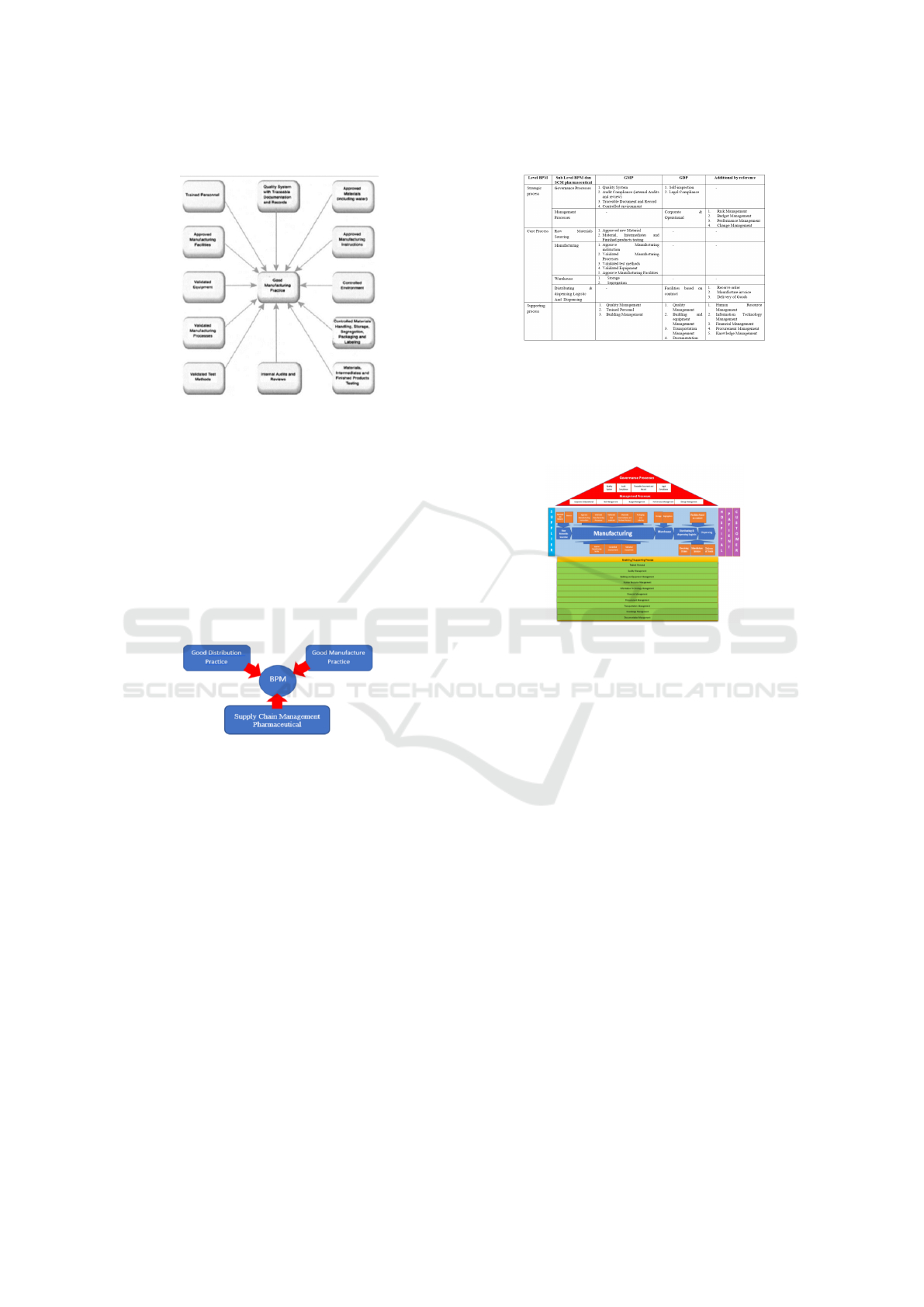
space
Figure 3: Good Manufacturing Practice (Van der Aalst,
2013)
3 METHOD
This research was conducted with a qualitative ap-
proach, which was carried out by reviewing the litera-
ture of several literary articles related to the subject of
this research and conducted a discussion forum with
the pharmaceutical industry in Indonesia. The follow-
ing research models can be seen in figure 4.
Figure 4: Research Model
The process carried out in Figure 4 is maps out
the processes that exist in GMP and GDP against the
levels that exist in the business process management
(BPM).
4 RESULTS AND DISCUSSION
This study analyzes the design of the BPM model
needed by companies or the pharmaceutical industry,
especially in Indonesia. According to John Jeston and
Johan Nelis(John, 2008) in the design of BPM, it is
necessary to consider the process architecture, which
is how to provide a process level description of the
business process that is occurring. In the process of
analyzing the BPM model by mapping out existing
processes according to the three BPM process levels,
the BPM process can be seen in Figure 5.
space
Figure 5: BPM Process Mapping GMP and GDP in Supply
Chain Management Pharmaceutical.
From the mapping process that is then carried out
by modelling the BPM design of the pharmaceutical
industry with combined GMP and GDP so that it can
be seen in Figure 6.
Figure 6: Business Process Management in the pharmaceu-
tical industry with Combination GMP and GDP
5 CONCLUSION AND FUTURE
RESEARCH
This study designed a business process management
model with a combination of GMP and GDP ap-
proaches to improve efficiency and effectiveness by
creating automation, speed, and process accuracy in
change management. This business process manage-
ment analyses existing performance processes then
makes changes when designing new processes by op-
timizing existing or available processes using vari-
ous facilities and methodologies. With this BPM
model, can improve business processes and supply
chain management processes in the pharmaceutical
industry, and can provide efficiency and effectiveness
in the industrial drug distribution process. With this, it
can reduce the problems in the pharmaceutical indus-
try in the circulation of medicines and the availability
of medicines in the market.
In the next stage, it is expected that it can validate
BPM for several existing industries, as well as be able
to measure the level of all the components of the BPM
model for the pharmaceutical industry.
CONRIST 2019 - International Conferences on Information System and Technology
182

ACKNOWLEDGEMENTS
Research supported by the Directorate General of
Strengthening for Research and Development, Min-
istry of Research, Technology, and Higher Edu-
cation, Republic of Indonesia is one part of the
results of Penelitian Dasar Unggulan Perguruan
Tinggi Research Grant to Binus University en-
titled “Pemanfaatan Technology Blockchain pada
management, pasok industry farmasi untuk men-
dukung Good Manufacturing Practice” or “Uti-
lization of Blockchain technology in pharmaceu-
tical industry supply chain management to sup-
port Integration of Good Manufacturing Practices”
with contract number: 225/SP2H/LT/DRPM/2019;
12/AKM/PNT/2019; 039/VR.RTT/IV/2019 and con-
tract date: 27 March 2019.
REFERENCES
Bitkowska, A. et al. (2018). Business process management
centre of excellence as a source of knowledge. Busi-
ness, Management and Education, 16(1):121–132.
Brocke, J., . M. J. (2018). Business process management
cases.
Buttigieg, S. C., Prasanta, D., and Gauci, D. (2016). Busi-
ness process management in health care: current chal-
lenges and future prospects.
Couckuyt, D. and Van Looy, A. (2019). Green bpm as
a business-oriented discipline: A systematic map-
ping study and research agenda. Sustainability,
11(15):4200.
Dachyar, M. and Novita, G. (2016). Business process
reengineering of logistics system in pharmaceutical
company. ARPN Journal of Engineering and Applied
Sciences, 11(7):4539–4546.
Fernando, E., Warnars, H. L. H. S., Kosala, R., Abdurach-
man, E., et al. (2018). Critical success factor of in-
formation technology implementation in supply chain
management: Literature review. In 2018 5th Interna-
tional Conference on Information Technology, Com-
puter, and Electrical Engineering (ICITACEE), pages
315–319. IEEE.
Gr
¨
une, G., Lockemann, S., Kluy, V., and Meinhardt, S.
(2014). Business Process Management Within Chem-
ical and Pharmaceutical Industries. Springer.
Hamilton, B. A. (2013). Implementing a pharmaceutical se-
rialization and traceability system in the united states.
Hein, T., Winkler, M., Gulati, H., and Balakrishnan, A.
(2015). Product lifecycle management for the phar-
maceutical industry. Oracle Life Sciences. Page-1-2.
Indonesia, P. R. (2009). Peraturan pemerintah nomor 51
tahun 2009 tentang pekerjaan kefarmasian. Jakarta:
Pemerintah RI.
John, J., . N. J. (2008). Business process manage-
ment: Practial guidelines to successful implementa-
tions (second edition).
Kitsios, F. and Kamariotou, M. (2019). Business strategy
modelling based on enterprise architecture: a state of
the art review. Business Process Management Journal.
Koster, S. R. (2009). An evaluation method for business
process management products. Master’s thesis, Uni-
versity of Twente.
Law, K. M. (2016). How schedule issues affect drug lo-
gistics operations: an empirical study in hospitals in
china. Industrial Management & Data Systems.
Malinova, M. and Mendling, J. (2018). Identifying do’s
and don’ts using the integrated business process man-
agement framework. Business Process Management
Journal.
Marcketti, S. B. (2005). Consumer concern, knowledge,
and attitude towards counterfeit apparel products: An
application of the theory of reasoned action theory.
Niehaves, B., Poeppelbuss, J., Plattfaut, R., and Becker, J.
(2014). Bpm capability development–a matter of con-
tingencies. Business Process Management Journal.
Nwabueze, U. (2012). Process improvement: the case of
a drugs manufacturing company. Business Process
Management Journal.
Puri, S. and Ranjan, J. (2012). Study of logistics issues
in the indian pharmaceutical industry. International
Journal of Logistics Economics and Globalisation,
4(3):150–161.
Ramos-Merino, M.,
´
Alvarez-Sabucedo, L. M., Santos-
Gago, J. M., and de Arriba-P
´
erez, F. (2019). A pattern
based method for simplifying a bpmn process model.
Applied Sciences, 9(11):2322.
Rentes, V. C., de P
´
adua, S. I. D., Coelho, E. B., Cintra, M.
A. d. C. T., Ilana, G. G. F., and Rozenfeld, H. (2019).
Implementation of a strategic planning process ori-
ented towards promoting business process manage-
ment (bpm) at a clinical research centre (crc). Busi-
ness Process Management Journal.
Rovers, J. P. and Mages, M. D. (2017). A model for a
drug distribution system in remote australia as a so-
cial determinant of health using event structure analy-
sis. BMC health services research, 17(1):677.
Shao, X. and Ji, J. (2006). Reconfiguration of pharmaceu-
tical logistics operations in china: an empirical study.
Transportation Journal, pages 52–66.
Smolnik, S., Urbach, N., Fjermestad, J. L., Cragg, P., and
Mills, A. (2011). It support for business processes in
smes. Business Process Management Journal.
Tipton, R. (2015). Industry overviews - pharmaceutical
management - research guides at rutgers university.
Tsakalidis, G., Vergidis, K., Kougka, G., and Gounaris, A.
(2019). Eligibility of bpmn models for business pro-
cess redesign. Information, 10(7):225.
Van der Aalst, W. M. (2013). Business process manage-
ment: a comprehensive survey. ISRN Software Engi-
neering, 2013.
Venkatraman, S. and Venkatraman, R. (2019). Process
innovation and improvement using business object-
oriented process modelling (boopm) framework. Ap-
plied System Innovation, 2(3):23.
Design Business Process Management Model for Pharmaceutical with Good Manufacture Practice and Good Distribution Practice in
Indonesia
183
