
Biodiesel Synthesis from Rubber Seed Oil via Esterification using
H-Zeolit and Zro
2
/ZAK Catalysts
Rahayu, Tiamina Nasution, Yunita Sari Lubis, Anggi Al Ridha Lubis, Agus Kembaren,
Rini Selly and Ahmad Nasir Pulungan
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Negeri Medan, Medan, Indonesia
Keywords: Biodiesel, Rubber Seed Oil, Zeolite, Heterogenous Catalysts
Abstract: Rubber seed oil is a non-edible oil that is very potential as a biodiesel feedstock. The synthesis of biodiesel
from rubber seed oil was done by reaction of esterification free fatty acid (FFAs) and transesterification of
Triglycerides (TGs) in a single step, which used heterogeneous catalysts. Zeolite is one type of catalyst that
has been developed for this process. In this study the catalyst was prepared using Indonesian natural zeolite
(ZAK). To increase catalytic activity, zeolite catalyst was loaded with zirconium (Zr) by wet impregnation
method, followed by the process of calcination and oxidation at a temperature of 400
o
C for 1 hours with gas
flow of O
2
and N
2
. ZAK and ZrO
2
/ZAK catalysts were characterized by XRD and SEM-EDS. The catalyst
produced has good crystallinity as seen from sharp peaks with high intensity and high levels of Si. The
catalyst activity test was done in the process of converting rubber seed oil into biodiesel. The reaction
process was carried out at a temperature of 100
o
C with a variety of catalyst concentrations: oil1: 2, 1: 4, and
1: 6 and the reaction time varied by 30, 60, and 90 minutes. The most optimum process of biodiesel
produced at the ratio of catalyst 1 : 2 with reaction time of 30 minutes showed the biodiesel yield of
67,95%. Biodiesel products were tested for water content, FFA, and density of each is 0,09%, 2,15%, dan
0,89 g/cm
3
. GC analysis shows that the main composition of biodiesel consists of diesel fraction (C
16
-C
20
)
with the most components being methyl linoleic 38,82% and methyl oleat 22,33%.
1 INTRODUCTION
Biodiesel is a renewable alternative fuel produced
from vegetable oils or animal fats. Vegetable and
animal oils are alternative energy sources that are
new, renewable and environmentally friendly in
addition to the fuels produced are also
biodegradeable and almost contain no sulfur and are
environmentally friendly (Jaya et al., 2011).
Vegetable oils developed in Indonesia are
sourced from palm oil, and jatropha oil, the current
research has reached the utilization of palm oil and
castor oil to substitute diesel fuel, but these materials
have limitations, palm oil is edible oils with high
selling value. According to Fukuda et al. (2001) and
Tyson (2004), edible oils as biodiesel feedstock
affect 60% -70% of biodiesel prices. Therefore, it is
open to find alternative energy sources from
vegetable oils with non-edible oil with abundant
availability and lower prices.
One source of vegetable oil that can be
developed is oil from rubber seeds. So far rubber
seeds have not been widely used and disposed of as
plantation waste. Meanwhile rubber seeds contain
about 40-50% of vegetable oil which is very
potential to be developed as raw material for
biodiesel synthesis (Setyawardhani et al, 2010). The
synthesis of biodiesel from rubber seed oil can be
done by an esterification reaction, where fatty acids
in rubber seeds will be reacted with short chain
alcohols resulting in fatty acid methyl esters. This
reaction is slow, so a catalyst is needed to reduce
activation energy and accelerate the reaction. This
reaction generally uses alkaline catalysts NaOH and
KOH.
However, rubber seed oil has a high content of
Free Fatty Acid (> 5%), the uses of alkaline catalysts
can cause saponification side reactions which can
reduce the rate of formation of biodiesel products.
Because it is currently being developed for this
process by using heterogeneous catalysts.
Rahayu, ., Nasution, T., Sari Lubis, Y., Al Ridha Lubis, A., Kembaren, A., Selly, R. and Pulungan, A.
Biodiesel Synthesis from Rubber Seed Oil Via Esterification using H-Zeolit and ZrO2/ZAK Catalysts.
DOI: 10.5220/0009873600002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 5-10
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5

The advantages of heterogeneous acid catalysts
are because they are less corrosive, do not need
expensive separation processes, and can reduce the
impact of environmental pollution (Leung et al.,
2009). One type of heterogeneous acidic material is
zeolite. The ability of zeolite as a catalyst is related
to the availability of active centers of Bronsted acid
sites and Lewis acid sites found in the channels
between zeolite (Sihombing et al., 2018; Sriningsih
et al., 2014; Pulungan, 2010). Modification of
zeolite has been carried out by impregnating metals
in carrier materials with the aim of prolonging the
life of the catalyst, having good thermal stability and
large surface area.
According to Sriatun and Suhartana (2002) the
metal which is applied to zeolite solids through
impregnation will make the metal in the zeolite as a
bifunctional catalyst. Heterogeneous catalysts that
are currently being developed for the production of
biodiesel are zeolite which is combined with metal
oxides such as ZrO2, SnO2. In addition, PbO and
ZnO metal oxides were also developed as catalysts
that are applied to zeolite. Singh et al., 2014 reported
that PbO / zeolite showed better activity than
ZnO/zeolite catalyst in the process of converting
sunflower oil with high free fatty acid content (>
10%). Meanwhile, Sukmawati (2016) has made
biodiesel from used cooking oil using sulfated
zirconia zeolite catalyst through a transesterification
reaction. From the results of this study, the
conversion of triglycerides was 71.63% at the
optimum condition of the reaction time for 120
minutes.
Therefore, in this study biodiesel synthesis from
rubber seed oil was carried out using active natural
zeolite catalyst which was treated with metal
Zirconium oxide (ZrO2 / ZAK). The uses of ZrO2 /
ZAK catalysts are expected to increase biodiesel
products and produce a cheaper and environmentally
friendly conversion process. In this study will be
studied the effect of catalyst concentration and
reaction time to obtain optimum catalyst activity.
2 MATERIALS AND METHOD
2.1 Tools
The tools used were a set of glass tools, reflux tools,
sokhlet tools, analytic balance, rotary evaporator,
buchner funnel, hotplate, thermometer, furnace,
magnetic stirrer, 100 mesh filter, porcelain cup,
oven, XRD, SEM-EDS, and GC.
The materials used were aquades, aquabides,
rubber seeds oil, commercial natural zeolites
(Bratachem), HCl (Merck pa), ZrCl
4
(Merck pa),
nitrogen gas, NaOH (Merck pa), H2SO4 (Merck pa),
PP indicators, AgNO
3
( Merck pa), H
3
PO
4
(Merck
pa), n-Hexane (technical), and CH
3
OH (Merck pa).
2.2 Preparation of Rubber Seed Oil
The rubber seeds were separated from the shell, then
the rubber seeds dried under the sun for 2-3 days
after that mashed using a blender. The refined rubber
seeds then extracted to obtain the oil with n-hexane
solvents at 60°C for 2 hours (5 cycles) continued
with separated the solvent using a rotary evaporator
to produce pure rubber seed oil, then analyzed to
determine the levels of free fatty acids (FFA ),
density and water content.
2.3 Preparation of Natural Zeolite
Natural zeolite was smoothed and filtered with a 100
mesh filter. Then washed with distilled water for 24
hours at room temperature. Zeolite filltered and the
precipitate dried at 100°C. The natural zeolite
sample was dehumuminated with 3M HCl and then
refluxed at 90°C for 30 minutes. The reflux solution
was filtered and the resulting sediment was washed
with distilled water until the pH of the washing
water was neutral. The precipitate was then dried at
120° C for 3 hours, then calcined at 400°C for 1
hour with gas nitrogen flow (± 20 mL / minute) to
obtain acid-activated natural zeolite (H-Zeolite)
(Sihombing et al. , 2018).
2.4 Preparation of ZrO2/ZAK Catalyst
An amount of 1% ZrCl
4
(% w Zr) was dissolved
with aquabides in a round bottom flask and then
added to ZAK, the mixture stirred for 3 hours at 80°
C after that zeolite was dried. Zeolite was then
oxidized with O2 gas at 400°C for 1 hour. The ZrO2
/ ZAK catalyst produced was characterized by FTIR
spectrometers Shimadzhu type 8201-FC, X-ray
difractometer Shimadzu 6100 using Cu Kα radiation
at 40 Kv and 30 mA with scanning rate of 2o min-1
in range 2θ 7
o
– 70
o
, SEM and SEM-EDS used Zeis
type EPOMH 10 Zss.
2.5 Synthesis of Biodiesel
Biodiesel made by mixing 99% methanol and zeolite
as a catalyst in a base flask with a ratio of zeolite: oil
that is 1: 2, 1: 4, and 1: 6 (v/v). The mixture then
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
6
added to rubber seed oil with a ratio of oil: methanol
1: 6 (v/v) and stirred at a speed of 600 rpm for 30,
60, and 90 minutes at 65°C at a pressure of 1 atm.
The mixture continued by filtered with a Buchner
funnel. Then decanted for 2 days. The biodiesel
produced was analyzed for FFA content, water
content, density, and GC characterization.
3 RESULTS AND DISCUSSION
3.1 Characterization of Natural
Zeolites
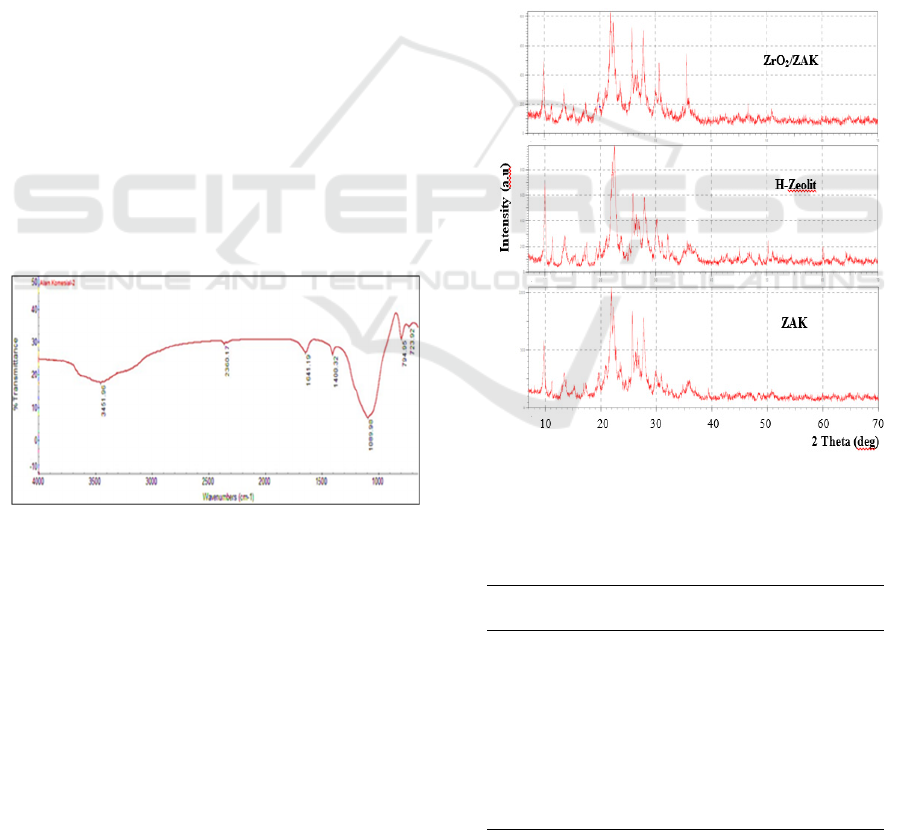
FTIR analysis was used to determine the functional
groups of zeolite samples. Based on the ZAK spectra
in figure 1 it can be seen that the TO
4
group
absorption characteristic of zeolite observed at
1089.98 cm-1. The absorption band of Si-O / Al-O
group at 723.92 cm
-1
and 794.95 cm
-1
. Hamdan
(1999) reported that in the 900-1250 cm
-1
band was
an asymmetrical range of the TO4 group, internal Si-
O / Al-O (TO) bending appeared in the 420-500 cm-
1 region while for the external would appear at 700-
780 cm
-1
(Hamdan, 1992). Sihombing et al (2018)
reported that asymmetrical range vibrations of the
TO4 zeolite group were in the range of1000-1100
cm
-1
. Asymmetric vibration absorption of the TO4
group from Sarulla zeolite was at 1042.16 cm
-1
(Nasution et al., 2019).
Figure 1: FTIR spectrum of commercial natural zeolite
Measurements with X-ray diffraction were
carried out to identify the crystallinity of the initial
natural zeolite, natural zeolite after acid activation
and after ZrO
2
was introduced. The results of XRD
analysis of each sample are presented in Figure 2.
Sharp peaks with high intensity show good
crystallinity. Figure 2 shows that the treatment of
HCl 3M (H-zeolite) activation provides an increase
in intensity on some of the main zeolite peaks at 20-
30 2 theta degrees. This is due to the loss of
amorphous and crystalline impurities that cover the
zeolite pores (Waluto et al., 2017; Sihombing et al.,
2018). In this figure ZrO
2
/ZAK also shows the
increasing intensity of the main zeolite peak. This
shows that the impregnation of ZrO
2
metal is
distributed evenly on the surface and pore of zeolite.
While the oxidation process at a high temperature of
500
o
C results in the loss of organic and inorganic
impurities in zeolite pores.
Based on table 1 Typical peaks of zeolite are seen
in H-Zeolite and ZrO
2
/ZAK at position 2 theta
degrees which are almost the same although with
slightly different intensities. This shows that acid
treatment is dealumination with 3M HCl in H-Zeolite
and thermal treatment ie oxidation on ZrO
2
/ ZAK
does not damage the structure of zeolite even though
there is a decrease in peak intensity of ZrO
2
/
ZAK. The decrease in peak intensity in metal-borne
natural zeolites proves that metals have been on the
surface of natural zeolites (Rianto et al., 2012).
Figure 2: Diffactogram comparison of ZAK, H-Zeolite
and ZrO
2
/ZAK
Table 1: The main peak intensity of ZAK, H-Zeolite and
ZrO
2
/ZAK
Catalyst 2𝜃
(°)
Intensity
(counts)
ZAK
9.87 364.90
22.54 548.03
35.74 142.15
H-Zeolite
10.04 304.2
21.99 623.52
35.87 142.01
ZrO
2
/ ZAK
9.03 302.21
27.7 527.17
35.53 256.24
Biodiesel Synthesis from Rubber Seed Oil Via Esterification using H-Zeolit and ZrO2/ZAK Catalysts
7
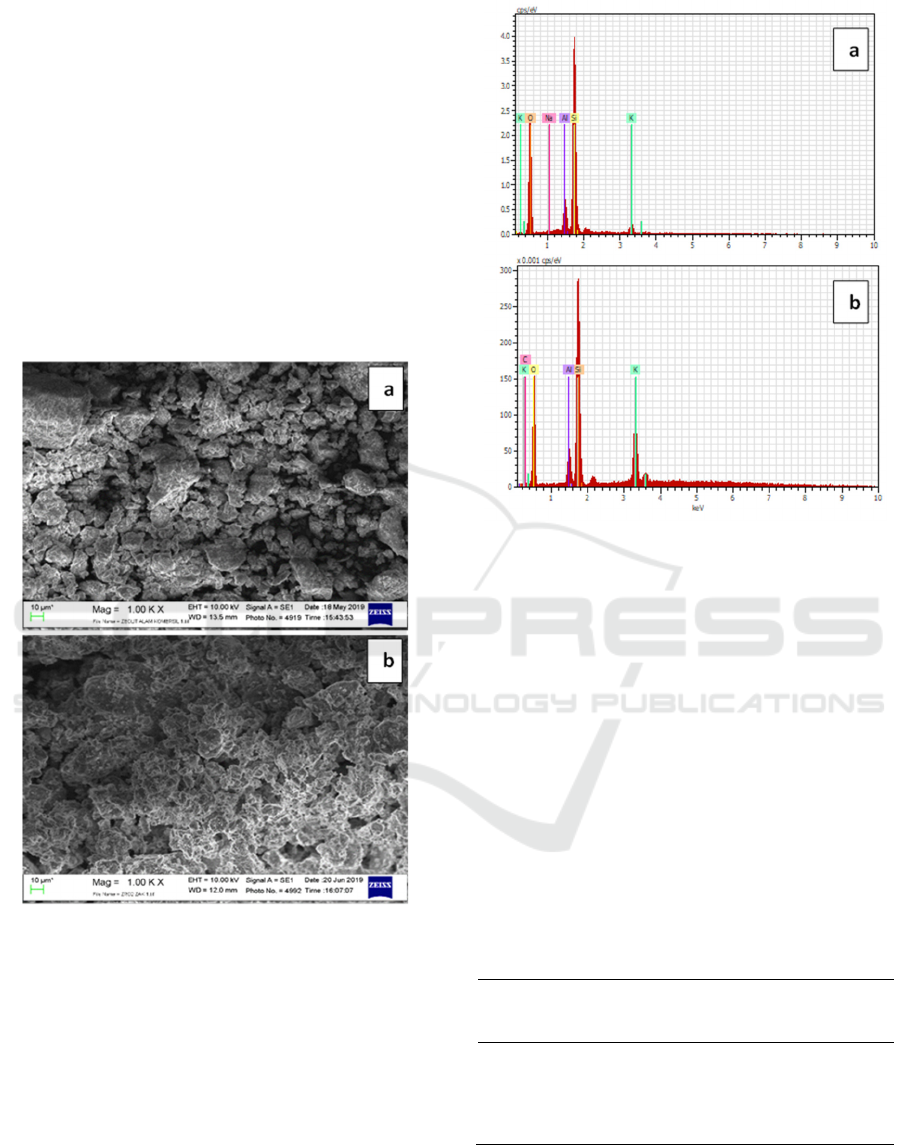
The SEM data showed information on surface
topology and metal dispersion that is applied to
zeolite, while from EDS data of chemical
composition is obtained on the surface of the
sample. Figure 3a and 3b describe surface topologies
of H-zeolite and ZrO2 / ZAK with a magnification
of 1000 times. In figure 3a the surface micrograph of
H-zeolite shows a surface structure consisting of
lamellar with a small size that is uneven and there
are still lumps. While ZrO2 / ZAK in figure 3b
shows a smooth and more homogeneous surface
structure. This data supports XRD data which shows
the metal dispersing process does not occur
sintering. Metal oxides are distributed evenly in
zeolite pores.
Figure 3: Surface topology enlarged 1000×. (a) natural
zeolite, (b) ZrO
2
/ZAK
Figure 4: Graph of chemical composition in (a) H-Zeolite,
(b) ZrO
2
/ZAK
3.2 Synthesis of Biodiesel from Rubber
Seed Oil
Rubber seed oil is obtained by soxhlet extraction
method using technical n-hexane solvents. The yield
of rubber seed oil obtained was 43.79%.
Characteristics of rubber seed oil based on practice
and standards are presented in table 2. Rubber seed
oil is obtained by soxhlet extraction method using
technical n-hexane solvents. The yield of rubber
seed oil obtained was 43.79%. Characteristics of
rubber seed oil based on practice and standards are
presented in Table 2.
Table 2: Characteristics of rubber seed oil (RSO) and
biodiesel
Characteristics
ASTM
D6751
(
biodiesel
)
RSO
Bio
Diesel
Water content
(
%
)
0.05 1.101 0.09
FFA (%) < 2 10.401 2.15
Density (g/cm
3
) 0.860-0.900 0.9097 0.89
The process of converting rubber seed oil with
ZAK and ZrO2 / ZAK catalysts is carried out by a
one-step process. In this reaction, esterification of
free fatty acids and transesterification of
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
8
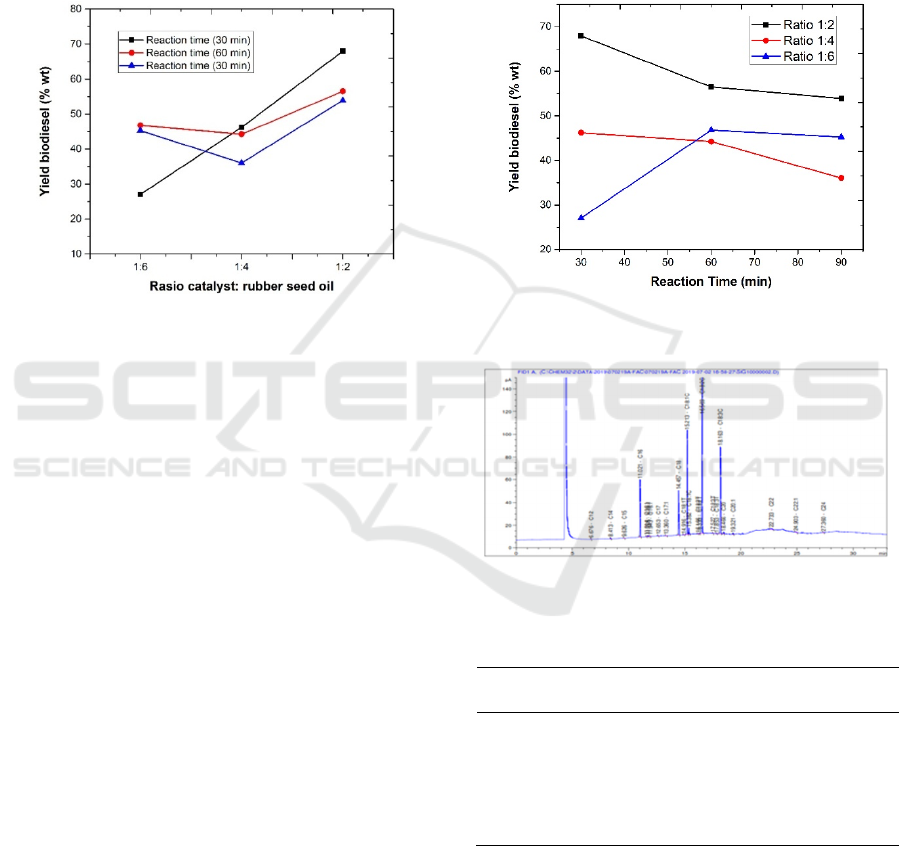
triglycerides occur the same process. The results of
the conversion of biodiesel products obtained are
presented in table 2. The optimum process
conditions were obtained at a catalyst ratio: 1: 2 and
a temperature of 30
o
C with a 67.95% biodiesel yield
value. To see the effect of variations in the catalyst
ratio: oil and process temperature, data made in
graphical form as shown in Figures 5 and 6. The
ratio of the catalyst: oil and reaction time affects the
conversion value of the biodiesel.
Figure 5: Graph of the relationship of reaction time to
biodiesel yield
In Figure 5, it can be seen that the most optimum
of reaction time which produces the highest yield is
30 minutes. In general, the longer the esterification
reaction time, the greater the conversion of oil to
biodiesel because of the greater the chance of
collisions between molecules occur. But at the
reaction time for 60 minutes and 90 minutes there
was a decrease in the yield of biodiesel produced,
this is because the reaction temperature used is close
to the boiling point of methanol (65
o
C) causing
some of the methanol to evaporate as the reaction
time increases. This resulted in a reduced molar ratio
of methanol to esterification oil. Tiamina et al.
(2019) reported that evaporation of methanol
resulted in a reduced molar ratio of methanol to
biodiesel production so that the yield of biodiesel
produced decreased.
Figure 6 shows that biodiesel yield increases
with increasing of catalyst:oil ratio. The increasing
of the concentration of the catalyst will reduce the
activation energy for the esterification reaction
thereby increasing the number of molecules
activated and reacting to form the fatty acid methyl
ester. At a reaction time of 30 minutes, a larger
catalyst: oil ratio shows a sharp increase in the
conversion of biodiesel products. Different trends
are shown at reaction times 60 and 90 minutes,
where the catalyst ratio increases: oil shows a
decrease in the conversion of biodiesel products and
shows an increase in a larger ratio. It can be
understood that at ratio: catalyst 1: 2 it is possible to
meet the active side of the catalyst with reactants
greater compared with the ratio of 1: 6. So that the
chance for contact between the reactants and the
catalyst to be greater so that it will produce a
catalyzed reaction that is also getting bigger. This is
indicated by the increasing conversion of biodiesel
products produced.
Figure 6: Graph of the relationship of ratio of rubber seed
oil to zeolite with the biodiesel yield
Figure 7: GC chromatogram of biodiesel product at
optimum condition.
Table 3: Chemical component of biodiesel
No. Component Methyl ester
Composition
(%)
1. C16 Methylpalmitate 9.80
2. C18 Methylstearate 8.85
3. C18 : 1 Methyloleate 22.33
4. C18 : 2 Methyllinoleic 38.82
5. C18 : 3 Methyllinolenic 18.22
6. C20 Methylarachidat 0.33
Figure 6 shows the chromatogram of biodiesel
products obtained at optimum conditions, with the
main content are methyl linoleic and methyl oleate
with a percentage of 38.82% and 22.33%
respectively.
Biodiesel Synthesis from Rubber Seed Oil Via Esterification using H-Zeolit and ZrO2/ZAK Catalysts
9

4 CONCLUSION
The acid activation process increases zeolite
crystallinity. The impregnated of zirconium oxide
(ZrO2) causes a shift and decreases the ripple
intensity of zeolite, but does not damage the zeolite
crystal structure. In the process of converting
biodiesel from rubber seed oil the optimum yield
was 67.95% at a catalyst ratio of 1: 2 with a reaction
time of 30 minutes. The main composition of
biodiesel consists of C16-C20 diesel fraction with
the most components, namely methyl linoleic (C18:
2) as much as 38.82% and methyl oleate (C18: 1) as
much as 22.33%.
ACKNOWLEDGEMENT
We would like to thanks Agency of the Ministry of
Research, Technology and Higher Education of the
Republic of Indonesia (KEMENRISTEKDIKTI)
which has funded our research via the Student
Creativity Program-Research (PKM-P) 2019.
REFERENCES
Fukuda , H., Kondo, A., dan Moda, H., 2001,
Biodisel Fuels Production
by
Transesterification of
Oils, J.Bio. Sci, Eng.,
405-416.
Hamdan, H., 1992, Introduction to Zeolites: Synthesis,
Characterization, and Modification, University
Teknologi Malaysia, Kuala Lumpur.
Jaya, N., danEthirajulu, K., 2011, Kinetics Modeling of
Transesterification Reaction for Biodisel Production
Using Heterogenous Catalyst, Inter. J of Eng. Sci. and
Tech. (IJEST), 3, 4, 3463- 3466.
Leung, E, Liu Y, Xuan W, MKH Leung., 2009 , A review
on Biodiesel Production Using Catalyzed
Transesterification, Applied Energi 87 (2010): 1083-
1095.
Nasution, T., Pulungan, A. M., Wiliranti, Y. A.,
Sihombing, J. L., & Pulungan, A. N. Synthesis of
Biodiesel From Rubber Seed Oil with Acid and Base
Activated Natural Zeolite Catalyst. Indonesian Journal
of Chemical Science and Technology (IJCST), 2(2),
125-130.
Pulungan, A. N. (2010). Preparasi dan karakterisasi katalis
NiO-CoO-MoO/Zeolit alam dan NiO-CoO-
MoO/Zeolit-Y untuk reaksi hidrorengkah minyak laka
menjadi fraksi bensin dan diesel (Doctoral dissertation,
Universitas Gadjah Mada).
Rianto, L.B., Amalia, S., and Khalifah,S.N.,2012,
Pengaruh Impregnasi Logam Titanium pada Zeolit
Alam Malang Terhadap Luas Permukaan Zeolit,
Alchemy 2(1):58-67.
Setyawardhani, D.A., S, Distantin., H, Henfiana., and A.S,
Dewi.,(2010), Pembuatan Biodiesel Dari
AsamLemakJenuhMinyakBijiKare,.
ProsidingSeminarRekayasa Kimia Dan Proses 2010,
Teknik Kimia UNDIP, Semarang.
Sihombing, J.L., Pulungan, A.N., Lindawati, P., Prayoga,
A., Safitri, I.A., Wandani, C.N., Silitonga,L.A.,
Ambarwati., Prayugo,P., and Wibowo, A.A., 2018,
Optimization of Indonesia Biodiesel Production from
Rubber Seed Oil Using Natural Zeolite Modification,
Jurnal Pendidikan Kimia 10(2) :387-392.
Sihombing, J. L., Gea, S., Pulungan, A. N., Agusnar, H.,
Wirjosentono, B., & Hutapea, Y. A. (2018, December).
The characterization of Sarulla natural zeolite crystal
and its morphological structure. In AIP Conference
Proceedings (Vol. 2049, No. 1, p. 020062). AIP
Publishing.
Sriatun and Suhartana., 2002, Impregnasi Nikel Klorida
pada Zeolit-Y untuk Katalis Hidrorengkah Minyak
Bumi Fraksi 150-230°C. FMIPA UNDIP.
Sriningsih, W., Saerodji, M. G., Trisunaryanti, W.,
Armunanto, R., &Falah, I. I. 2014. Fuel production
from LDPE plastic waste over natural zeolite supported
Ni, Ni-Mo, Co and Co-Mo metals. Procedia
Environmental Sciences, 20, 215-224.
Sukmawati,P.D., 2016, Optimization of Used Cooking Oil
Into Biodiesel with Sulfated Zirconia Zeolit Catalyst,
prosiding Seminar Nasional Teknik Kimia. ISSN 1693-
4393.
Trisunaryanti, W., 2016, Material Katalis dan
Karakternya, UGM PRESS, Yogyakarta.
Tyson, K., 2009, Biodisel Handling and Use Guideline,
National Renewble
Energy
Laboratory,Mild
West.
Waluyo, J., T. Richards, I. G. B. N. Markertihartha and
Susanto, H., 2017, Asia-Pacific Journal of Chemica
Engineering 17(1), 37-45
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
10
