
Relationship between Environmental Factors and Rheumatic Heart
Disease
Tina Christina Lumban Tobing
1*
, Teddy Ontoseno
2
, Sri Endah Rahayuningsih
3
, Ratna Akbari Ganie
4
,
Yahwardiah Siregar
5
1
Department of Child Health, Medical School, Universitas Sumatera Utara
2
Department of Child Health, Medical School, Universitas Airlangga
3
Department of Child Health, Medical School, Universitas Padjajaran
4
Department of Clinical Pathology, Medical School, Universitas Sumatera Utara
5
Biochemistry Department, Medical Faculty, Universitas Sumatera Utara
Keywords: children, environmental, rheumatic heart disease
Abstract: Rheumatic fever and rheumatic heart disease are the most common acquired heart diseases in children and
the most common cause of death in the field of pediatric cardiology. Environmental factors play an
important role in RHD. The prevalence of RF and RHD tend to decrease as socioeconomic status improves.
To determine the relationship between environmental factors and RHD in children. A case-control study
was conducted in the Department of Child Health, Haji Adam Malik Hospital from April to June 2017. The
case group has consisted of children aged 5-18 years with RHD while the control group has consisted of
healthy children. Demographic, anthropometric, and laboratory data were collected along with
environmental factors. Statistical analysis was done using Statistical Product and Service Solution (SPSS).
A P value of <0,05 at 95% confidence interval was considered significant. A total of 39 children were
enrolled in each group. Subjects’ median age was 13.0 years. Males were dominant compared to females.
Fathers who went to elementary and junior high school had a higher risk of having children with RHD (OR
28; P value 0,032 and OR 15,75; P value 0,011, respectively). Mothers who went to junior high school had
7 times higher risk of having children with RHD (P-value 0,026). Low monthly income increased the risk of
RHD (OR 3,68; P value 0,009). Tap water usage, meat consumption more than once per week, and
feasibility to buy clothes more than 1 pair per year decreased the risk of RHD at 0,31 (P value 0,013), 0,3
(P-value 0,016), and 0,04 times (P-value <0,001), respectively. Parent’s education, monthly family income,
water source, consumption of meat, and feasibility to buy clothes are related to RHD in children.
1 INTRODUCTION
Rheumatic fever (RF) and rheumatic heart
disease (RHD) are the most common acquired heart
diseases in children and the most common cause of
death in the field of pediatric cardiology. Rheumatic
heart disease causes permanent damage to heart
valve tissue and in a chronic condition may lead to
congestive heart disease, stroke, endocarditis, and
death (Park, 2008; Seckeler, 2011). A study in India
reported an annual mortality rate in children from
RF and RHD as high as 3,3%. The estimation of
annual death from RF and RHD in Asia lied between
356.000 and 524.000 (Carapetis, 2008; Kumar,
2002).
Rheumatic fever and RHD are complex diseases
influenced by genetic, the virulence of bacteria, and
environmental factors (Guilherme, 2012).
Environmental factors such as overcrowding and
lack of ventilation play important role in RHD. The
prevalence of RF and RHD tend to decrease as
socioeconomic status improves (Ibrahim-Khalil,
1992; Grover, 1993, Cheng, 2009). Low
socioeconomic status is marked with a high poverty
level, low educational status, high illiteracy rate, and
high unemployment rate. Those are related to RF
and RHD even though direct relationships have not
been proven at present (Kerdemelidis, 2010; Steer,
2002; Vlajinac, 1989; Vlajinac, 1991).
Rheumatic fever and RHD can be prevented with
primary and secondary prophylaxis. Primary
180
Tobing, T., Ontoseno, T., Rahayuningsih, S., Ganie, R. and Siregar, Y.
Relationship between Environmental Factors and Rheumatic Heart Disease.
DOI: 10.5220/0009862901800184
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 180-184
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
prophylaxis for children with pharyngitis is carried
out with a well-planned program along with
sanitation, health infrastructure, and socioeconomic
status improvements. This is proven to reduce the
incidence of RF in Australia, Sweden, and the USA
(Carapetis, 2005; Seckeler, 2011).
Our objective is to determine the relationship
between environmental factors and RHD in children
in Medan, Indonesia.
2 METHOD
An observational case-control study was
conducted in the Department of Child Health, Haji
Adam Malik hospital Medan from April to June
2017. Subjects were obtained using consecutive
sampling method. Children aged 5-18 years and
diagnosed with RHD were enrolled in case of the
group while the control group has consisted of
healthy children. Demographic and anthropometric
data were collected along with laboratory results.
Environmental factors were obtained from each
patient and his/her parents including parents'
education, monthly family income, water source,
frequency of meal, consumption of meat, feasibility
to buy clothes, house's dweller, household's fuel, and
house's profiles. All subjects underwent
echocardiography evaluation. Children with
congenital heart disease were excluded from this
study. Informed consent was obtained before
conducting the procedure. This study was approved
by the Health Research Ethical Committee, Medical
School, Universitas Sumatera Utara.
Statistical analysis was done using computer
software. Chi-square and Fisher's exact tests were
used to analyze the relationship between categorical
variables. Mann-Whitney tests were used to
determine the relationship between categorical and
continuous variables. The analysis was conducted at
a 95% confidence interval and a P value of <0,05
was considered significant.
3 RESULTS AND DISCUSSION
Rheumatic heart disease is caused by an
immunologic response toward Streptococcus
pyogenes infection. The infection usually manifests
as tonsillopharyngitis. The M protein from bacteria's
cell wall has a similar structure (molecular mimicry)
with several proteins in heart valve tissue. The
molecular mimicry triggers an autoimmune reaction
and causes tissue damage (Guilherme, 2005).
A total of 78 subjects were enrolled in this
study. Male subjects were dominant with the median
age of 13,0 years (range 5,0-18,0 years). Most
subjects had normal antistreptolysin titer O (ASTO)
and C-reactive protein (CRP) levels. A study in 2012
showed a similar result to our study. The highest
prevalence of RHD was observed in children aged 5-
16 years, followed by children aged more than 16
years. They found no RHD case in children aged
under 5 years (Prajapati, 2013). Rheumatic fever and
RHD rarely occurred before 4 years old and even
rarer before 2 years of age. The underlying cause of
this condition is that the peak incidence of
tonsillopharyngitis occurs between 5-15 years of age
(Anderson, 2010). There was no gender predilection
in RHD (Anderson, 2010) as observed in this study.
Baseline characteristics of subjects were described
in Table 1.
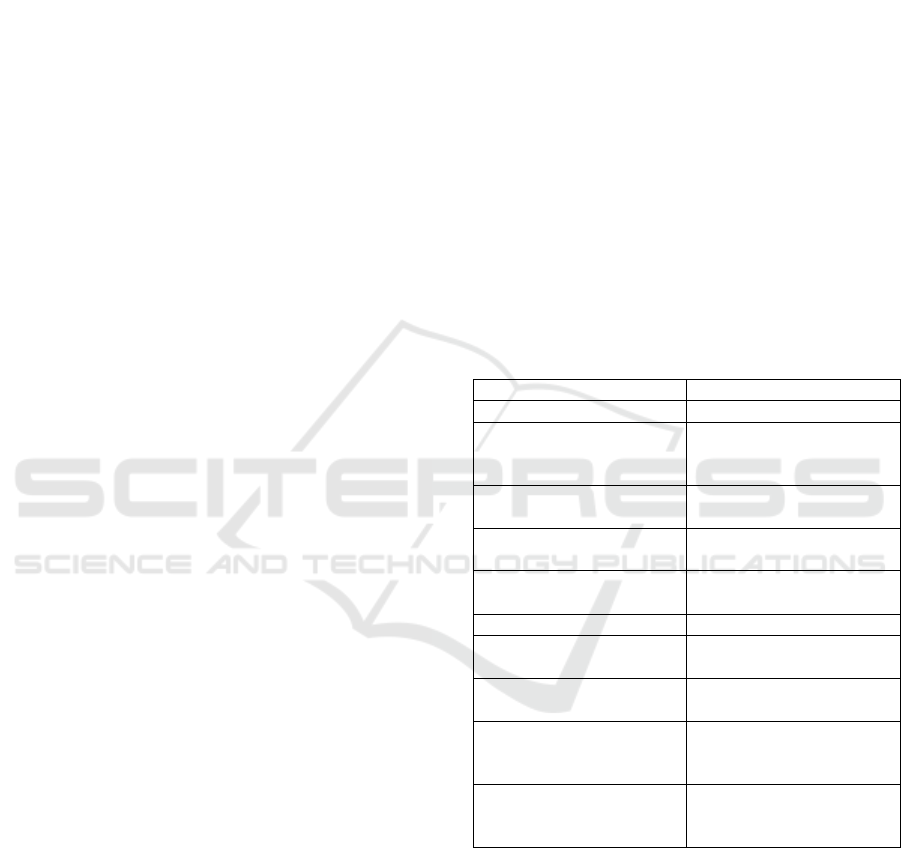
Table 1: Baseline characteristics of subjects.
Characteristics n=78
Median age, year (range) 13,0 (5,0-18,0)
Gender, n (%)
Male
Female
43 (55,1)
35 (44,9)
Mean body weight, kg
(SD)
31,6 (10,61)
Median body height, cm
(range)
139,0 (60,0-173,0)
Mean hemoglobin level,
g/dL (SD)
11,5 (1,74)
Mean hematocrit, % (SD) 35,4 (5,30)
Median leukocyte level,
mL
-1
(range)
9.905,0 (3.900,0-
23.690,0)
Mean thrombocyte level,
mL
-1
(SD)
336.346,2 (125.053,2)
ASTO level, n (%)
≤200 IU
>200 IU
50 (64,1)
28 (35,9)
CRP level, n (%)
≤0.7 mg/L
>0.7 mg/L
52 (66,7)
26 (33,3)
Most subject’s parents went to senior high
school and had monthly income at or lower than
minimum regional standard. Median subject’s house
size was 60,0 m
2
with median house’s dwellers of
5,0 persons. Distribution of environmental factors as
described in Table 2.
Relationship between Environmental Factors and Rheumatic Heart Disease
181
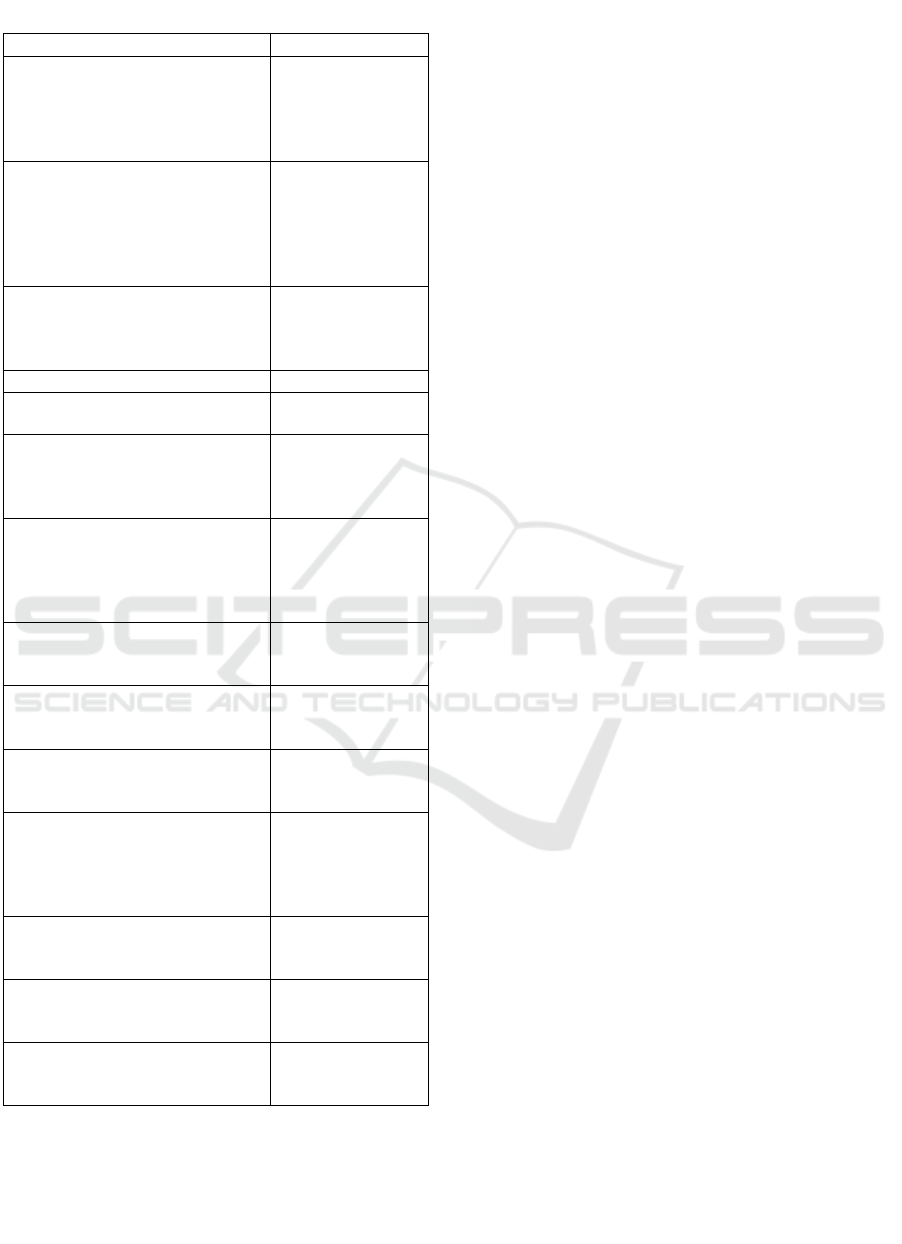
Table 2: Distribution of subjects’ environmental factors.
Characteristics n=78
Father’s education, n (%)
University
Senior high school
Junior high school
Elementary school
8 (10,3)
39 (50,0)
26 (33,3)
5 (6,4)
Mother’s education, n (%)
University
Senior high school
Junior high school
Elementary school
No formal education
9 (11,5)
32 (41,0)
30 (38,5)
5 (6,4)
2 (2,6)
Monthly family income, n (%)
> minimum regional standard
≤ minimum regional standard
27 (34,6)
51 (65,4)
Median house size, m
2
(range) 60,0 (24,0-180,0)
Median house’s dwellers, person
(range)
5,0 (2,0-7,0)
House floor material, n (%)
Bamboo
Cement
Ceramic
2 (2,6)
57 (73,1)
19 (24,4)
House wall material, n (%)
Palm leaves
Wood
Brick
Wall
2 (2,6)
7 (9,0)
17 (21,8)
52 (66,7)
Latrine possession, n (%)
Yes
No
76 (97,4)
2 (2,6)
House’s electricity, n (%)
Available
Not available
78 (100,0)
0 (0,0)
Water source, n (%)
Well
Tap water
37 (47,4)
41 (52,6)
Household’s fuel, n (%)
Firewood
Charcoal
Kerosene
Gas
3 (3,8)
3 (3,8)
15 (19,2)
57 (73,1)
Consumption of meat, n (%)
Once/week
> once/week
52 (66,7)
26 (33,3)
Feasibility to buy clothes, n (%)
1 pair/year
> 1 pair/year
40 (51,3)
38 (48,7)
Frequency of meal, n (%)
< 3 times daily
≥ 3 times daily
7 (9,0)
71 (91,0)
Parent's education, monthly family income,
water source, consumption of meat, and feasibility to
buy clothes were related to RHD in this study.
Fathers who went to elementary and junior high
school had a higher risk of having children with
RHD at 28,0 times (P-value 0,032) and 15,75 times
(P-value 0,011), respectively compared to fathers
who went to university. Mothers who went to junior
high school also had 7,0 times higher risk of having
children with RHD (P-value 0,026). Lower monthly
family income would increase the risk of RHD.
Family with monthly income at or lower than
minimum regional standard had 3,68 times higher
risk of having children with RHD (P-value 0,009)
compared to a family with monthly income higher
than minimum regional standard. These findings are
confirmed by several studies. A study in 2005
showed that poverty, overcrowding, and lower
parent’s education were risk factors of RHD (Meira,
2005). Improvement in socioeconomic status was
related to decreasing RHD prevalence in North India
(Negi, 2013).
Consumption of meat and feasibility to buy
clothes also affected the incidence of RHD. Risk of
RHD was lower in a family which able to consume
meat more than once per week (OR 0,3; P value
0,016) and which able to buy clothes more than 1
pair per year (OR 0,04; P-value <0,001) compared to
their counterparts (Table 3). These variables
represent a family's socioeconomic status. The more
frequent consumption of meat in a family and the
more clothes a family can afford, the better it's
socioeconomic status. The better socioeconomic
status allows the family to fulfill adequate nutritional
support, complete access to healthcare facility
including immunization, and good housing. These
factors play an important role in preventing
streptococcal tonsillopharyngitis as the preceding
event of RHD (Feikin, 2009; Abdullah, 2010).
Worse housing quality and low socioeconomic
status would increase the susceptibility of RHD
according to a study by Dobson, et al (Dobson,
2011). In our study, a family which used tap water
as the water source was less likely to have children
with RHD (OR 0,31; P value 0,013). Families which
can afford tap water pipeline generally have better
housing quality and socioeconomic status. Better
housing quality ensures good hygiene and prevents
the incidence and transmission of
tonsillopharyngitis.
We found no relationship between overcrowding
and RHD in this study. This is in contrast with
several other studies. Okello stated that the risk of
RHD was increased in the overcrowded population
(Okello, 2012). A similar result was reported by
Jaine (Jaine, 2011). The different result may be
caused by the high population in Indonesia so that
the number of house's dwellers were similar between
case and control groups.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
182
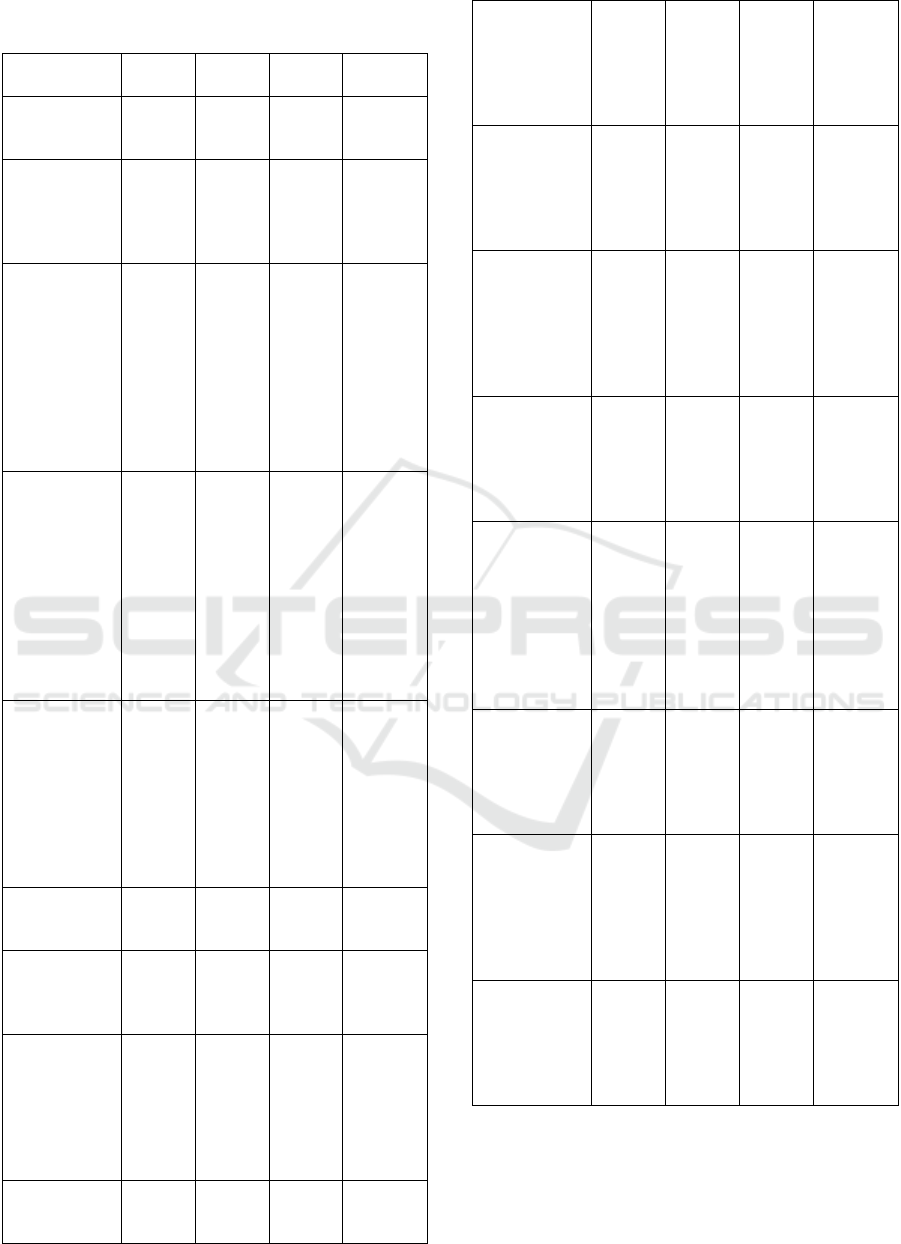
Table 3: Relationship between demographic and
environmental factors and RHD.
Factors RHD No
RHD
OR 95% CI
Median age,
year (range)
13,0
(5,0-
18,0)
13,0
(5,0-
18,0)
N/A
N/A
Gender, n (%)
Male
Female
21
(53,8)
18
(46,2)
22
(56,4)
17
(43,6)
1,109
b
0,454-
2,708
Father’s
education, n
(%)
University
Senior high
school
Junior high
school
Elementary
school
1 (2,6)
12
(41,0)
18
(46,2)
4 (10,3)
7(17,9)
23
(59,0)
8 (20,5)
1
(2,6)
Ref
4,870
c
15,750
c
*
28,000
c
*
Ref
0,545-
43.523
1,652-
150.141
1,350-
580.591
Mother’s
education, n
(%)
University
Senior high
school
Junior high
school
Elementary
school
None
2 (5,1)
12
(30,8)
20
(51,3)
4 (10,3)
1 (2,6)
7(17,9)
20
(51,3)
10
(25,6)
1 (2,6)
1 (2,6)
Ref
2,100
c
7,000
c
*
14,000
c
3,500
c
Ref
0,374-
11.807
1,222-
40.089
0,944-
207.597
0,145-
84.694
Monthly
family
income, n (%)
> minimum
regional
standard
≤ minimum
regional
standard
31
(79,5)
8 (20,5)
20
(51,3)
19
(48,7)
3,681
b
*
1,355-
9,998
Median house
size, m
2
(range)
60,0
(24,0-
180,0)
48,0
(25,0-
144,0)
N/A
a
N/A
Median
house’s
dwellers,
person (range)
5,0
(2,0-
7,0)
5,0
(3,0-
7,0)
N/A
a
N/A
House floor
material, n
(%)
Bamboo
Cement
Ceramic
1 (3,1)
31
(96,3)
7
(87,5)
1 (3,7)
26
(96.3)
12
(92,3)
Ref
1,192
c
0,583
c
Ref
0,071-
20,011
0,031-
10,863
House wall
material, n
(%)
2(40,0)
0 (0,0)
Ref
Ref
Palm leaves
Wood
Brick
Wall
3
(60,0)
10
(83.3)
24
(92,3)
4
(100,0)
7
(100,0)
28
(100,0)
N/A
c
N/A
c
N/A
c
N/A
N/A
N/A
Latrine
possession, n
(%)
Yes
No
39
(100,0)
2 (5,1)
37
(94,9)
0 (0,0)
N/A
c
N/A
House’s
electricity, n
(%)
Available
Not
available
39
(100,0)
0 (0,0)
39
(100,0)
0 (0,0)
N/A
N/A
Water source,
n (%)
Well
Tap water
24
(61,5)
15
(61,5)
13
(33,3)
26
(66,7)
0,313
b
*
0,124-
0,790
Household’s
fuel, n (%)
Firewood
Charcoal
Kerosene
Gas
2 (5,1)
1 (2,6)
12
(30,8)
24
(61,5)
1 (2,6)
2 (5,1)
3
(7,7)
33
(84,6)
Ref
0,250
c
2,000
c
0,364
c
Ref
0,008-
7,542
0,133-
30,162
0,031-
4,245
Consumption
of meat, n (%)
Once/week
> once/week
8 (20,5)
31
(79,5)
18
(46,2)
21
(538)
0,301
b
*
0,111-
0,819
Feasibility to
buy clothes, n
(%)
1 pair/year
> 1 pair/year
6 (15,4)
33
(84,6)
32
(82,1)
7 (17,9)
0,040
b
*
0,012-
0,131
Frequency of
meal, n (%)
< 3 times
daily
≥ 3 times
daily
5 (12,8)
34
(87,2)
2 (5,1)
37
(94,9)
0,368
c
0,067-
2,021
Mann Whitney test
b
Chi square test Fischer's exact test *P<0,05
Our study had several limitations. There is no
strict classification of socioeconomic status in
Indonesia so we only gathered factors which
influence poverty based on Badan Pusat Statistik
criteria (Badan Pusat Statistik, 2017). We did not
Relationship between Environmental Factors and Rheumatic Heart Disease
183

match subjects in case and control groups and this
might cause selection bias. Our data was also not
normally distributed, preventing us to perform a
parametric study. Further study enrolling more
subjects from several centers is needed to confirm
the result of this study.
4 CONCLUSION
Parent’s education, monthly family income, water
source, consumption of meat, and feasibility to buy
clothes are related to RHD in children. Lower
parent’s education and monthly family income will
increase the susceptibility of having children with
RHD. Tap water usage, frequent consumption of
meat, and feasibility to buy more clothes are
protective factors of RHD.
ACKNOWLEDGMENTS
The researchers are grateful to all subjects and their
families and to all persons/institution who support
our study.
REFERENCES
Abdullah, A., Clark, A., Longenecker, J.C., Whitty, C.J.,
2010. Physical accessibility and utilization in Yemen.
Int J Health Geogr. 9:38.
Anderson, R.H., 2010. Pediatric Cardiology: rheumatic
fever, 3rd edition, Churchill Livingstone, Philadelphia.
Susanti, N., 2017. Analisis indikator kemiskinan
Kabupaten Demak tahun 2017, ed Nugroho, B.A.
Badan Pusat Statistik Kabupaten Demak, Demak,
p.12-4.
Carapetis, J.R., 2008. Rheumatic heart disease in Asia.
Circulation. 118:2748-53.
Carapetis, J.R., Steer, A.C., Mulholland, E.K., Weber, M.,
2005. The global burden of group A streptococcal
diseases. Lancet Infect Dis. 5:685-94.
Cheng, T.O., 2009. How much of the recent decline in
rheumatic heart disease in China can be explained by
changes in cardiovascular risk factors?. Int J Cardiol.
132:300-2.
Dobson, J., Steer, A.C., Colquhoun, S., Kado, J., 2011.
Environmental factors and rheumatic heart disease in
Fiji. Pediatr Cardiol. 33:332-6.
Feikin, D.R., Nguyen, L.M., Adazu, K., Ombok, M., Audi,
A., 2009. The impact of distance of residence from
peripheral health facility on pediatric health utilization
in rural western Kenya. Trop Med Int Health. 14:54-61.
Grover, A., Dhawan, A., Iyengar, S.D., Anand, I.S., Wahi,
P.L., 1993. Epidemiology of rheumatic fever and
rheumatic heart disease in a rural community in
northern India. Bull World Health Organ. 71:59-66.
Guilherme, L., Kohle, K.F., Kalil, J., 2012. Rheumatic
heart disease: genes, inflammation, and autoimmunity.
Rheumatol Cur Res. 6:1066-9.
Guilherme, L., Oshiro, S., Fae, K., Kalil, J., 2005.
Molecular pathogenesis of rheumatic heart disease.
Expert Review in Molecular Medicine. 69:1-15.
Ibrahim-Khalil, S., Elhag, M., Ali, E., Mahgoub, F.,
Hakiem, S., 1992. An epidemiological survey of
rheumatic fever and rheumatic heart disease in sahafa
town Sudan. J Epidemiol Community Health. 46:477-9.
Jaine, R., Baker, M., Venugopal, K., 2011. Acute
rheumatic fever associated with household crowding
in a developed country. Pediatr Infect Dis J. 30:315-9.
Kerdemelidis, M., Lennon, D.R., Arroll, B., Peat, B.,
Jarman, J., 2010. The primary prevention of rheumatic
fever. J Paediatr Child Health. 46:534-48.
Kumar, R., Raizada, A., Aggarwal, A.K., Ganguly, N.K.,
2002. A community-based rheumatic fever/ rheumatic
heart disease cohort: twelve-year experience. Indian
Heart J. 54:54 –8.
Meira, Z.M.A., Goulart, EMA., Colosimo, E.A., Mota,
C.C.C., 2005. Long term follows up of rheumatic
fever and predictors of severe rheumatic valvular
disease in Brazilian children and adolescents. Heart.
91:1019-22.
Negi, P.C., Kanwar, A., Chauhan, R., Sanjeev, A., Thakur,
J.S., Bhardwaj, A.K., 2013. Epidemiological trends of
RF/RHD in school children of Shimla in north India.
Indian J Med Res. 137:1121-7.
Okello, E., Kakande, B., Sebatta, E., Kayima, J., Kuteesa,
M., Mutatina, B., et al, 2012. Socioeconomic and
environmental risk factors among rheumatic heart
disease patients in Uganda. PLoS ONE. 7:e43917.
Park, M.K., 2008, Acute rheumatic fever. In: Paediatric
Cardiology for Practitioners, 5th Edition, ed Park,
M.K. Mosby Elsevier, Philadelphia, p.604-13.
Prajapati, D., Sharma, D., Regmi, P.R., Khanal, H.,
Baidya, S.G., Rajbhandari, S., Rokka, M., et al, 2013.
Epidemiological survey of rheumatic fever, rheumatic
heart disease and congenital heart disease among
school children in Kathmandu valley of Nepal. NHJ.
1:1-10.
Seckeler, M.D., 2011. The worldwide epidemiology of
acute rheumatic fever and rheumatic heart disease.
Clin Epidemiol. 3:67-84.
Steer, A.C., Carapetis, J.R., Nolan, T.M., Shann, F., 2002.
A systematic review of rheumatic heart disease
prevalence in children in developing countries: the
role of environmental factors. Circulation. 38:229-34.
Vlajinac, H., Adanja, B., Jarebinski, M., 1989.
Socioeconomic factors and rheumatic fever
occurrence. Difference between patients with and
without a frequent sore throat. J Hyg Epidemiol
Microbiol Immunol. 33:471-6.
Vlajinac, H., Adanja, B., Marinkovic, J., Jarebinski, M.,
1991. Influence of socio-economic and other factors
on rheumatic fever occurrence. Eur J Epidemiol.
7:702-4.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
184
