
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin
Coconut Oil in Streptozotocin-induced Diabetic Rats
Linda Margata
1*
, Jansen Silalahi
1
, Urip Harahap
2
, Dwi Suryanto
3
and Denny Satria
4
1
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Universitas Sumatera Utara, Medan, Indonesia
2
Department of Pharmacology, Faculty of Pharmacy, Universitas Sumatera Utara, Medan, Indonesia
3
Department of Biology, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan, Indonesia
4
Department of Pharmaceutical Biology, Faculty of Pharmacy, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Virgin coconut oil, Enzymatic hydrolysis, Blood glucose, Haemoglobin A1c, Superoxide dismutase
Abstract: The aim of this study was to examine the antidiabetic and antioxidant effect of enzymatically hydrolyzed
virgin coconut oil (HVCO) in streptozotocin (STZ) induced rats. VCO was hydrolyzed enzymatically using
lipase from Rhizomucor miehei (active on sn-1,3 position). Thirty male rats were induced with 40 mg/kg
body weight (BW) STZ. Rats with blood glucose level ≥ 250 mg/dl were divided into six groups which
were given with sodium carboxymethylcellulose (CMC Na) 0.5%, metformin 45 mg/kgBW, VCO (4 and 6
ml/kgBW) and HVCO (4 and 6 ml/kgBW). Blood glucose, Haemoglobin A1c (HbA1c), superoxide
dismutase (SOD), soluble receptor advanced glycosylation end-product (sRAGE) levels, and
immunihistochemistry assay on pancreas were analyzed after 30 days of treatment. It is shown that HVCO 4
ml/kgBW and metformin were not significantly different in lowering blood glucose level. Blood glucose
level in groups treated with HVCO 4 ml/kgBW and metformin were 409.2 and 364.40 mg/dl. HVCO also
lowered HbA1c and sRAGE levels (55.50 ng/ml and 148.40 pg/ml, respectively), while increased SOD
level (76.96 pg/ml). Insulin expression of rats treated with 4 ml/kgBW HVCO and metformin also did not
differ significantly which were 11.40% and 11.70%, respectively. HVCO exerted higher antidiabetic and
antioxidant effects that VCO did.
1 INTRODUCTION
Diabetes Mellitus (DM) lowers life’s quality,
productivity and increases mortality rate in either
developing or developed countries. DM is metabolic
syndrome which is marked by the occurence of
hyperglycemia. Hyperglycemia in DM could trigger
oxidative stress which then causes microvascular
complications (retinopathy, nephropathy and
neuropathy) and also macrovascular complications
(heart attack, stroke and peripheral blood vessel
disease). Risk of heart attack and stroke happen two
to four times more in diabetic patients, 50% diabetic
patients died because of cardiovascular disease
(Vassalotti, 2006; Paliyath, et al., 2011).
Insulin plays an important role in controlling
blood glucose. Insulin is a hormon produced by β-
cell which is located in Langerhans islet of pancreas.
Insulin stimulates the uptake of blood glucose by
cell, hence lowers blood glucose level. DM which is
caused by the low secretion of insulin from β-cell
pancreas is classified as Type I DM, while DM
which is caused by insulin resistance is classified as
Type II DM (Joshi, et al., 2007; Triplitt, et al.,
2005).
Hyperglycemia stimulates protein glycosylation
which is a reaction between aldehyde group in
glucose and amino group in protein. This reaction
produces schiff base via Amadori process. Amadori
product then undergoes further autooxidation
becomes advanced glycosylation end-product
(AGE). Crosslinking protein causes AGE
accumulation in extracellular matrix. AGE in
diabetic patient causes erythrocyte dysfunction
because haemoglobin is glycated. This erythrocyte
will bound to receptor of advanced glycosylation
end-product (RAGE) in endothelium and causes
endothelium dysfunction (Baynes and
Domonickzak, 2003; McKee and McKee, 2003;
Rayfield and Valentine, 2006; Mechanick, 2006).
Virgin coconut oil (VCO) is one of the sources
of medium chain triglyceride (MCT) oil. MCT has
152
Margata, L., Silalahi, J., Harahap, U., Suryanto, D. and Satria, D.
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin Coconut Oil in Streptozotocin-induced Diabetic Rats.
DOI: 10.5220/0009862301520159
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 152-159
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

been known to have benefits in glycemic control and
insulin secretion. MCT helps in the effectiveness of
glucose usage (Vala and Kapadiya, 2014; Bach and
Babayan, 1982; Fife, 2004). Studies about the
antidiabetic and antioxidant effects of VCO has been
reported (Siddalingaswamy, et al., 2011;
Mohammed and Luka, 2013; Iranloye, et al., 2013;
Akinnuga, et al., 2014; Kolondam, et al., 2008;
Elsayed, et al., 2015; Essien, et al., 2014;
Dalmacion, et al., 2012; Dewi and Aryadi, 2010).
However, study about the effect of hydrolyzed VCO
(HVCO) on blood glucose level and its antioxidant
effect have never been reported. The objective of
this study was to determine the antidiabetic and
antioxidant effects of HVCO in STZ-induced
diabetic rats.
2 MATERIALS AND METHODS
Apparatus used in this study were analytical balance,
hot plate, magnetic stirrer, thermometer, oven, water
bath, separating funnel, Easytouch test strips,
microtube, centrifugator, microplate reader,
incubator, micro pipette and laboratory glassware.
Chemicals used were lipase from Rhizomucor
miehei (≥ 20000 U/g, Sigma Aldrich), Tris-HCl
(molecular biology grade, Vivantis), distilled water
(Bratachem), n-hexane (Macron Chemicals).
Calcium chloride and sodium sulfate anhydrous
were the products of Merck. All reagents used in this
work were of analytical grade unless otherwise
stated. VCO used was the product of Palem Mustika,
Indonesia. STZ used to induce diabetic in rats was
from Nacalai Tesque. Metformin 500 mg tablets
were purchased from the local pharmacy. Rat
HbA1c, SOD and sRAGE ELISA kits were the
products of FineTest.
2.1 Enzymatic Hydrolysis of Virgin
Coconut Oil
Thirty (30) g of VCO, 30 ml of distilled water, 12.5
ml of 0.063 M calcium chloride, 25 ml of buffer
Tris-HCl 1 M pH 8 and 3 ml of lipase from R.
miehei were transferred into 250 ml Erlenmeyer
flask, respectively. The mixture was incubated for
10 hours at 50°C and stirred at 200 rpm for 10
minutes every 1 hour. After 10 hours, the mixture
was transferred into the separating funnel and
extracted with 50 ml of n-hexane. The extract was
allowed to stand for a while until two layers were
formed. The upper layer (n-hexane fraction) was
separated (filtrate I), while the bottom layer (water
fraction) was extracted again with 50 ml of n-
hexane. The second extract was allowed to stand for
a while and then the upper layer formed was
separated (filtrate II). Filtrate I and II were mixed
and then 250 g of sodium sulfate anhydrous was
added and allowed to stand for 15 minutes to absorb
the water residue. The mixture was filtered, then n-
hexane was evaporated using water bath to obtain
HVCO (Margata, et al., 2018).
2.2 Preparation of Diabetic Rats
Thirty male rats which had been acclimatized for
one week were fasted for 18 hours and water was
given ad libitum during fasting period. Blood
glucose level was measured using Easytouch test
strips. Rats were given STZ 40 mg/kgBW i.p. and
then 10% sucrose ad libitium for one day. After 10
days, blood glucose level was measured and rats
with blood glucose level ≥ 250 mg/dl were used as
experimental animals (Furman, 2015).
2.3 Experimental Design
Diabetic rats were divided into six groups (five
animals each) and were given orally: (1) CMC Na
0.5% (control group), (2) 45 mg/kgBW metformin,
(3) 4 ml/kgBW VCO, (4) 6 ml/kgBW VCO, (5) 4
ml/kgBW HVCO, and (6) 6 ml/kgBW HVCO, for
30 days. Blood glucose level was measured every
three days during experimental period. After the end
of treatment, rats were fasted for 18 h and
anesthetized with 70 mg/kgBW ketamine i.p. Blood
was collected directly from the heart and centrifuged
for 10 min at 3000 rpm to obtain serum. Serum
collected was then analyzed for HbA1c, SOD and
sRAGE levels using ELISA (Afriadi, 2010; Silalahi,
et al., 2016).
2.4 HbA1c, SOD and sRAGE Analysis
HbA1c, SOD and sRAGE were measured
enzymatically using ELISA kit. Absorbance of color
intensity was read using microplate reader at 450 nm
(Ashraf, et al., 2015).
2.5 Immunohistochemical Staining of
Pancreas
Insulin expression in pancreas was done using IHC
technique. Pancreas tissue which had been sectioned
into 3-4 µm was soaked intu xylol, ethanol 100, 90,
80, 70 and 50%, respectively, each was done 2 times
for 90 min. Peroxidase blocking was done using
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin Coconut Oil in Streptozotocin-induced Diabetic Rats
153
0.3% H
2
O
2
in methanol for 20 min, washed with
10% phosphat buffer saline (PBS) 3 times for 5 min.
Non-specific blocking was done using 10% normal
serum for 30 min and then tissue was incubated with
primary antibody at 4°C for 18-22 hrs, washed with
10% PBS 3 times for 5 min. Tissue was given with
some drops of secondary (universal) antibody and
allowed to stand for 30 min and then washed with
10% PBS 3 times for 5 min. Tissue was given with
some drops of chromogen 3,3-diaminobenzidine and
allowed to stand for 5-10 sec, then washed with
distilled water, counterstained with Hematoxylin
Mayer for 5-10 sec and then with running tap water
for 10-15 min. Dehydration was done using ethanol
80, 90% and xylol (each for 2 times). Mounting was
done using E. Z mount (Lab Vision, Cat#MS-1378-
PO)
2.6 Statistical Analysis
All data were statistically analyzed using one-way
ANOVA followed by Tukey’s test using
computerized SPSS package program (SPSS 17.0
software for Windows). Results are expressed as
mean±standar deviation and considered significantly
different at p<0.05.
3 RESULTS
The effect of VCO and HVCO on blood glucose
level during 30 days of treatment can be seen in
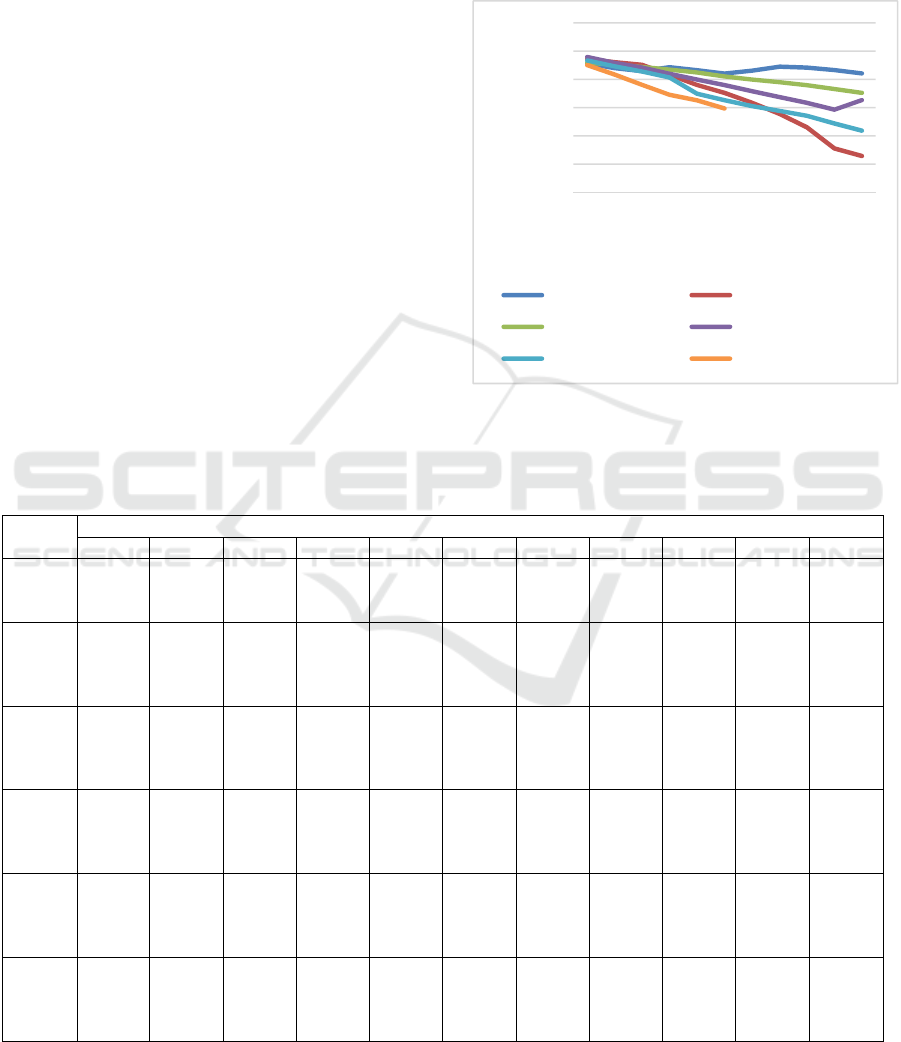
Table 1 and Figure 1.
Figure 1: Changes in blood glucose level during 30 days
of treatment.
Table 1: Changes in blood glucose level during 30 days of treatment
Treat-
ment
Blood glucose level (mg/dl) day-
0 3 6 9 12 15 18 21 24 27 30
Negati
ve
control
528.40
±
18.56
a
519.80
±
14.79
a
514.60
±
19.86
a
521.60
±
12.10
a
516.20
±
17.51
a
510.40
±
14.93
a
515.40
±
11.30
a
522.40
±
11.01
a
520.80
±
14.86
a
516.40
±
15.65
a
510.60
±
15.95
a
Metfor
-min
536.00
±
47.72
a
530.60
±
46.77
a
525.80
±
45.50
a
508.00
±
32.44
a
490.20
±
29.16
a
476.20
±
23.81
a,
b
458.60
±
26.75
a,
b
439.00
±
19.34
b
415.20
±
16.39
b
377.80
±
16.90
b
364.40
±
11.50
b
VCO 4
ml/kg
BW
529.40
±
31.93
a
526.00
±
31.46
a
522.00
±
32.02
a
517.40
±
30.01
a
512.20
±
30.43
a
505.20
±
31.33
a,
b
499.80
±
34.13
a,
b
495.00
±
33.59
a,
c
489.80
±
33.81
a,
c
483.00
±
33.13
a,
c
476.20
±
32.84
a,
c
VCO 6
ml/kg
BW
539.60
±
30.76
a
529.20
±
31.54
a
521.00
±
33.58
a
510.00
±
32.27
a
499.80
±
31.32
a
490.00
±
33.66
a,
b
479.00
±
31.02
a,
b
468.60
±
32.36
a,
b,c
458.40
±
32.12
b,
c
446.40
±
27.52
c,
d
463.40
±
29.37
c,
d
HVCO
4
ml/kg
BW
533.60
±
36.58
a
523.00
±
36.82
a
514.00
±
34.16
a
503.40
±
38.04
a
474.60
±
41.60
a
463.20
±
41.79
a,
b
452.80
±
40.31
b
444.20
±
39.13
b,
c
435.60
±
38.47
b,
c
422.00
±
40.85
b,
d
409.20
±
46.15
b,
d
HVCO
6
ml/kg
BW
525.20
±
29.45
a
508.40
±
27.05
a
490.40
±
29.52
a
472.80
±
29.66
a
463.00
±
29.80
a
448.40
±
35.83
b
- - - - -
Means ± SD in each column with different superscript letters differ significantly at p<0.05 (n=5).
300
350
400
450
500
550
600
036912151821242730
Blood glucose level (mg/dl)
Day-
Negative control Metformin
VCO 4 ml/kgBW VCO 6 ml/kgBW
HVCO 4 ml/kgBW HVCO 6 ml/kgBW
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
154
In Table 1 and Figure 1, it can be seen that at day
0, all rats in each group had blood glucose level ≥
250 mg/dl. Blood glucose levels at day 0 in all rats
were not significantly different. From day 3 to 30,
blood glucose levels in all groups, except negative
control, were gradually decreasing. In negative
control group, blood glucose level was stable until
day 30. From day 3 to 12, all all groups had
significantly different blood glucose level (p>0.05).
At day 15, blood glucose levels in rats given with
metformin and HVCO 6 ml/kgBW were 476.20 and
448.40 mg/dl, respectively. Those values were
significantly different with blood glucose level in
negative control group which was 510.40 mg/dl. At
day 18, HVCO 4 ml/kgBW was shown to have a
significant decrease in blood glucose level compared
to negative control group. At day 30, blood glucose
level in rats fed with 4 ml/kgBW VCO did not differ
significantly (p<0.05) compared to blood glucose
level in rats fed with metformin which were 409.20
and 364.40 mg/dl.
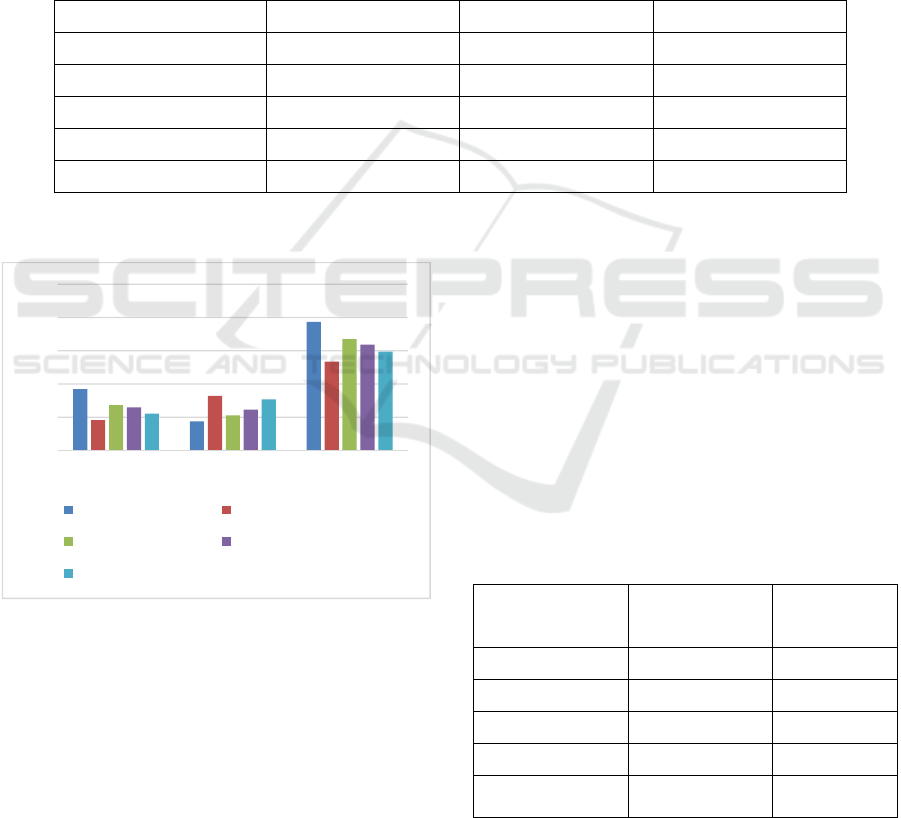
The effect of VCO and HVCO on HbA1c, SOD
and sRAGE levels in diabetic rats can be seen on
Table 2 and Figure 2.
Table 2: The effect VCO and HVCO on HbA1c, SOD and sRAGE levels in diabetic rats
Treatment HbA1c (ng/ml) SOD (pg/ml) sRAGE (pg/ml)
Negative control
92.45 ± 1.44
a
43.95 ± 0.58
a
193.49 ± 2.53
a
Metformin
45.75 ± 0.77
b
82.15 ± 0.39
b
133.82 ± 1.63
b
VCO 4 ml/kgBW
68.38 ± 0.73
c
52.82 ± 0.22
c
167.55 ± 2.53
c
VCO 6 ml/kgBW
64.88 ± 1.41
d
61.49 ± 0.50
d
159.30 ± 1.93
d
HVCO 4 ml/kgBW
55.50 ± 1.59
e
76.96 ± 0.32
e
148.80 ± 1.99
e
Means ± SD in each column with different superscript letters differ significantly at p<0.05 (n=5).
Figure 2: The effect of VCO and HVCO on HbA1c,
SOD and sRAGE levels in diabetic rats.
As seen in Table 2 and Figure 2, HbA1c levels in
all groups were significantly different (p<0.05). The
highest to the lowest HbA1c levels started from
negative control, 4 ml/kgBW VCO, 6 ml/kgBW
VCO, 4 ml/kgBW HVCO to metformin,
respectively. SOD levels in all groups were also
significantly different (p<0.05). The lowest SOD
level was in negative control group which was 43.95
pg/ml, while the highest was in metformin group
which was 82.15 pg/ml. SOD level in rats fed with 4
ml/kgBW HVCO (52.82 pg/ml) was significantly
higher than those in 4 and 6 ml/kgBW VCO (52.82
and 61.49 pg/ml). All groups also had significantly
different sRAGE levels (p<0.05). The highest
sRAGE level was in negative control group, while
sRAGE level in groups fed with metformin, 4
ml/kgBW HVCO, 4 and 6 ml/kgBW VCO were
shown to decrease significantly compared to
negative control group.
The effect of VCO and HVCO on insulin
expression in pancreas of diabetic rats can be seen
on Figure 3 and Table 3.
Table 3: The effect VCO and HVCO on insulin expression
in diabetic rats
Treatment Mean insulin
expression ±
SD
Insulin
expression
(%)
Negative control 6,40 ± 1,82
a
3.20
Metformin 23,40 ± 1,14
b
11.70
VCO 4 ml/kgBW 10,60 ± 1,82
c
5.30
VCO 6 ml/kgBW 15,60 ± 1,67
d
7.80
HVCO 4
ml/kgBW
22,80 ± 1,48
b
11.40
Means ± SD in each column with different superscript
letters differ significantly at p<0.05 (n=5). Percentage of
insulin expression was calculated from 200 cells which
was counted using Image Raster
0
50
100
150
200
250
HbA1c (ng/ml) SOD (pg/ml) sRAGE (pg/ml)
Negative control Metformin
VCO 4 ml/kgBW VCO 6 ml/kgBW
HVCO 4 ml/kgBW
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin Coconut Oil in Streptozotocin-induced Diabetic Rats
155
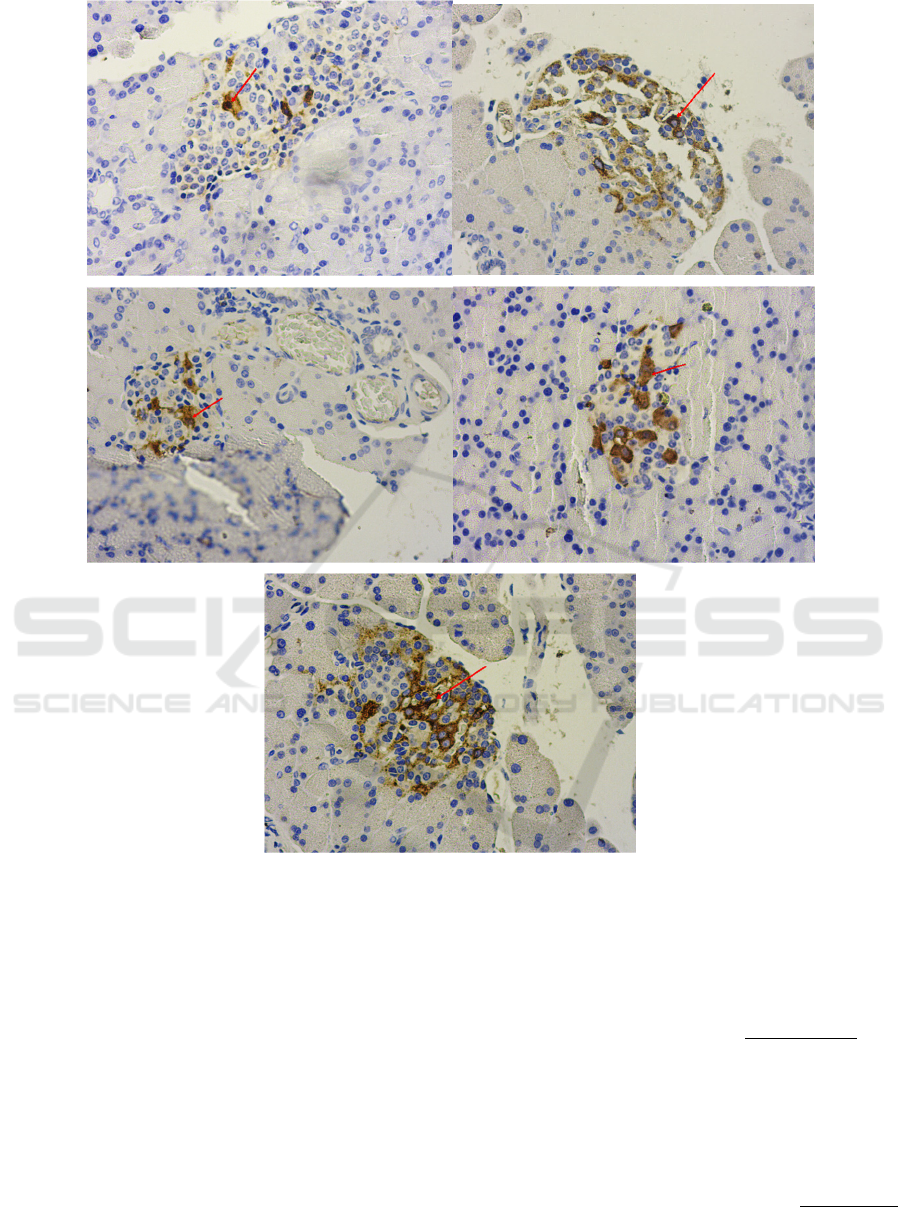
Figure 3: The effect of VCO and HVCO on insulin expression in diabetic rats. A = negative control, B = metformin 45
mg/kgBW, C = VCO 4 ml/kgBW, D = VCO 6 ml/kgBW, E = HVCO 4 ml/kgBW, (→) = show positive reaction between
antigen and insulin antibody on β-cell which is marked by brown color; magnificent 10 x 40.
Based on Figure 3, it can be seen that rats from
negative control had the least brown color expressed
compared to other groups. In addition, it is also
shown that rats treated with metformin and 4
ml/kgBW HVCO showed highest insulin expression.
From Table 3, it can be seen that insulin expression
in negative control group was the lowest insulin
expression with score 6.40 (3.20%). Insulin
expression in each group differs significantly at
p<0.05, except groups treated with metformin and
HVCO 4 ml/kgBW with insulin expression of 27,60
(13,80%) and 22.80 (11.40%), respectively.
4 DISCUSSION
In this study, 40 mg/kgBW STZ was shown to give
uniform response of blood glucose levels in all
groups. STZ is a broad spectrum antibiotic which is
toxic to insulin producing β-cell in pancreas
Langerhans islet. STZ is uptaken via glucose
transporter GLUT2 and causes DNA alkylation, and
eventually causes β-cell death (Szkudelski, 2011;
Deeds, et al., 2011). β-cell damage was shown to be
stable until day 30 in this study. Blood glucose level
A
B
C
D
E
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
156

was not return to normal during experiment period if
intervention was not done. Structural changes in β-
cell pancreas (total granulation) occur 48 hours after
STZ administration and continue for 4 months. The
low rate of β-cell regeneration in diabetic individual
causes the importance of finding a way to increase
its regeneration rate (Eleazu, et al., 2013; Yin, et al.,
2006).
Blood glucose level was decrease after 15 day of
treatment in groups fed with metformin and 6
ml/kgBW. It shows that the bioavailability of
metformin and 6 ml/kgBW HVCO were enough to
decrease blood glucose level in diabetic rats after 15
days. After day 15, blood glucose level in group fed
with 6 ml/kgBW was not recorded because all rats in
those group did not survive until day 18. Therefore,
HbA1c, SOD and sRAGE levels in that group were
not available.
VCO was shown to have antidiabetic effect and
the effect increasing as the dosage increasing. This
study corresponds to the studies reported before
which showed that antidiabetic effect of VCO is
dose dependant (Iranloye, et al., 2013; Afriadi, 2010;
Handajani and Dharmawan, 2009). At day 30, group
fed with 4 ml/kgBW and metformin were shown to
have a decrease in blood glucose level. It might be
because those groups undergo β-cell recovery at day
30. MCFA contained in HVCO is suspected to have
important role in the recovery of β-cell pancreas. In
addition, lauric acid also has the ability to stimulate
insulin secretion (Vala and Kapadiya, 2014).
Metformin is an antihyperglycemic commonly
used in the treatment of Type II DM. Metformin
increases hepatic and peripheral insulin sensitivity
by inhibiting hepatic glucose production and
increasing the uptake of glucose in skeletal muscle
and adipose. Although metformin was not
commonly recommended as adjunct therapy in Type
I DM (Beysel, et al., 2018), this study shows that
metformin was able to lower blood glucose level in
single dose STZ induced diabetic and this finding
corresponds to the works reported before (Silalahi,
et al., 2016; Erehywa, et al., 2011; Han, et al., 2017).
This might be because diabetic type induced by STZ
has similarity either with Type I or Type II DM
(Eleazu, et al., 2013).
VCO enzymatic hydrolysis using lipase from
Rhizomucor miehei which is active on sn-1 and sn-3
position in triglyceride molecule produces two
MFAs (especially lauric acid) and one molecule 2-
monoglyceride (especially 2-monolaurin) (Aehle,
2004). MCFA is known to help blood glucose
control, increase insulin secretion and help in
glucose usage, hence MCFA can be used in diabetic
prevention and treatment (8,10). In β-cell, MCFA
activates fatty acid 1 receptor (FFAE1/GPR40) and
induces mitochondira ketogenesis, hence increases
β-cell function (Pujol, et al., 2018).
VCO and HVCO was shown to decrease HbA1c
levels although the decrease were not more effective
than that of metformin. HVCO lowered HbA1c
levels better than VCO. HbA1c is an indicator used
to monitor diabetic condition and hyperglycemia.
Glycemic control helps in decreasing the risks of
heart failure, myocardial infarction, etc. Recent
study shows that high HbA1c involved in the
increase risk of cardiovascular disease mortality in
diabetic patients (Wong, et al., 2018).
HVCO was shown to helps in decreasing
excessive oxidative stress condition in diabetic rats,
hence, it prevented the occurence of DM
complications. This result corresponds to the studies
reported before that VCO increases SOD level in
diabetic rats (Siddalingaswamy, et al, 2011;
Iranloye, et al., 2013). Oxidative stress is known to
be the key role of diabetic complications
pathogenesis. Human body is constantly protected
from excessive oxidative stress by a complex system
of enzymatic and non-enzymatic antioxidant.
Enzymatic antioxidant SOD involves in reactive
oxygen species (ROS) metabolsim. Superoxide
anion is highly reactive ROS which is converted by
SOD into hydrogen peroxide which is then reduced
to water by catalase and gluthation peroxidase
(Wong, et al., 2018; Ng, et al., 2013).
HVCO and VCO decreased sRAGE in diabetic
rats, although it was not more effective than
metformin. RAGE is a cell surface type receptor
from immunoglobulin superfamily which binds to
various ligands, including AGE. Soluble RAGE
(sRAGE) is a RAGE isoform found in blood
circulation. The binding of ligand and sRAGE
prevents the condition of oxidative stress,
inflammation and apoptosis which occur from the
interaction between RAGE and ligand. In
hyperglycemia condition, ROS and AGE induces
metalloproteinase-9 matrix which cleaves the cell
surface receptor which produces sRAGE, hence
increasing sRAGE levels in DM patients (Wong, et
al., 2018).
Antidiabetic drug metformin is known to
ameliorates oxidative stress status in DM.
Metformin prevents SOD inhibition caused by
aldehyde modification and increase its antioxidant
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin Coconut Oil in Streptozotocin-induced Diabetic Rats
157

activity in diabetic patient. In addition, metformin
also prevents oxidative stress by decreasing ROS
and increasing enzymatic antioxidant. DM condition
causes proteins to undergo non-enzymatic glycation
with reducing sugar and produces AGE. Production
of AGE is followed by oxidative reaction which
produces radical compounds. The interaction
between AGE and its receptor (RAGE) involves in
the development of microvascular and
macrovascular complication (Ng, et al., 2013).
In this study, it was found that rats given with
VCO showed increased insulin expression compared
to rats in control group, although the score was not
higher than in metformin group. However, HVCO
showed insulin expression which was not
significantly different from metformin. This finding
corresponds to the work reported before which VCO
was able to increase β-cell and serum insulin in
diabetic rats (Iranloye, et al., 2013).
VCO and HVCO had the ability to inhibit the
continuous damage of β-cell in Langerhans islet.
This might be caused by their abilities to decrease
oxidative stress which happens in diabetic condition.
MCFAs like lauric, palmitic and capric acid which
contained in VCO and HVCO are able to increase
insulin secretion by releasing intracellular calcium
from calcium channel in β-cell membrane plasma.
Increase in insulin secretion causes lower production
of free radicals, hence decrease β-cell damage. In
addition, VCO also contains vitamin E and
antioxidants which are able to neutralize free
radicals accumulatted in diabetic condition
(Supriatna, et al., 2018).
5 CONCLUSION
HVCO is more effective than VCO in lowering
blood glucose, HbA1c and sRAGE levels, while
increasing SOD level and insulin expression in
diabetic rats. Blood glucose, HbA1c, SOD, sRAGE
levels and insulin expression in metformin and 4
ml/kgBW HVCO groups were not significantly
different after 30 days of treatment, hence, HVCO is
effective as antidiabetic and antioxidant in STZ-
induced diabetic rats.
ACKNOWLEDGEMENTS
If any, should be placed before the references
section without numbering.
REFERENCES
Aehle, W., 2004. Enzyme in Industry, Wiley-VCH.
Weinheim.
Afriadi, A., 2010. Uji Efek Virgin Coconut Oil (VCO)
terhaap Berat Badan dan Penurunan Kadar Gula
Darah (KGD) Tikus Putih Diabetes yang diinduksi
Streptozotocin (STZ). Tesis. Program Studi Magister
dan Doktor Ilmu Farmasi. Fakultas Farmasi.
Universitas Sumatera Utara.
Akinnuga, A.M., Jeje, S.O., Bamidele, O. and Sunday,
V.E., 2014. Dietary consumption of virgin coconut oil
ameliorates lipid profiles in diabetic rats. Physiology
Journal, 2014.
Ashraf, J.M., Ahmad, S., Choi, I., Ahmad, N., Farhan, M.,
Tatyana, G. And Shahab, U., 2015. Recent advances
in detection of AGEs: Immunochemical, bioanalytical
and biochemical approaches. IUBMB Life, 67(12),
pp.897-913.
Bach, A.C. and Babayan, V.K., 1982. Medium-chain
triglycerides: an update. The American Journal of
Clinical Nutrition, 36(5), pp.950-965.
Baynes, J. and Domonickzak, M.H. 2003. Medical
Biochemistry, Elsevier Science Mosby. London.
Beysel, S., Unsal, I.O., Kizilgul, M., Caliskan, M., Ucan,
B. and Cakal, E., 2018. The effects of metformin in
type 1 diabetes mellitus. BMC Endocrine Disorders,
18(1), p.1.
Dalmacion, G.V., Ortega, A.R., Pena, I.G. and Ang, C.F.,
2012. Preliminary study on the in-vitro susceptibility
of Mycobacterium tuberculosis isolates to virgin
coconut oil. Functional Foods in Health and Disease,
2(8), pp.290-299.
Deeds, M.C., Anderson, J.M., Armstrong, A.S., Gastineau,
D.A., Hiddinga, H.J., Jahangir, A., Eberhardt, N.L.
and Kudva, Y.C., 2011. Single dose streptozotocin-
induced diabetes: considerations for study design in
islet transplantation models. Laboratory Animals,
45(3), pp.131-140.
Dewi, S.S. and Aryadi,T., 2010. Efektifitas virgin coconut
oil (VCO) terhadap kandidiasis secara in vitro. In
Prosiding Seminar Nasional & Internasional.
Eleazu, C.O., Eleazu, K.C., Chukwuma, S. and Essien,
U.N., 2013. Review of the mechanism of cell death
resulting from streptozotocin challenge in
experimental animals, its practical use and potential
risk to humans. Journal of Diabetes and Metabolic
Disorders, 12(1), p.60.
Elsayed, H.H., Elrahman, M.K.A., Emara, A.H. and El-
Hafez, A., 2015. Compare effect of fatty acid
composition (olive, coconut oil and butter) on adipose
liver tissue, and serum lipid profile in albino rats.
IOSR-JBB, 1, pp.28-38.
Erejuwa, O.O., Sulaiman, S.A., Ab Wahab, M.S.,
Sirajudeen, K.N.S., Salleh, M.S.M. and Gurtu, S.,
2011. Glibenclamide or metformin combined with
honey improves glycemic control in streptozotocin-
induced diabetic rats. International Journal of
Biological Sciences, 7(2), pp.244.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
158

Essien, N.M., Bassey, S.C., Nna, V.U. and Ofem, O.E.,
2014. Comparative effect of chronic consumption of
some edible vegetable oils on lipid profile and some
haematological parameters in rats. Annals of
Biological Research, 5(7), pp.16-21.
Fife, B., 2004. The Coconut Oil Miracle, Penguin Group.
USA.
Furman, B.L., 2015. Streptozotocin-induced diabetic
models in mice and rats. Current Protocols in
Pharmacology, 70(1), pp.5-47.
Han, X., Tao, Y.L., Deng, Y.P., Yu, J.W., Cai, J., Ren,
G.F., Sun, Y.N. and Jiang, G.J., 2017. Metformin
ameliorates insulitis in STZ-induced diabetic mice.
PeerJ, 5, p.e3155.
Handajani, N.S. and Dharmawan, R., 2009. Effect of VCO
to leucocyte differential count, glucose levels and
blood creatinine of hyperglycemic and ovalbumin
sensitized Mus musculus Balb/c. Bioscience, 1(1),
pp.1-8.
Iranloye, B., Oludare, G and, Olubiyi, M., 2013. Anti-
diabetic and antioxidant effects of virgin coconut oil in
alloxan induced diabetic male sprague dawley rats.
Journal of Diabetes Mellitus, 3(4), p.221-226.
Joshi, S.R., Parikh, R.M. and Das, A.K., 2007. Insulin –
history, biochemistry, physiology and pharmacology.
Supplement of JAPI, 55, p.19-25.
Kolondam, B.J., Pokatong, W. And Tallei, T., 2008. Kadar
trigliserida dan kolesterol tikus wistar (Rattus
novergicus) setelah konsumsi virgin coconut oil.
Biosaintifika, 1, pp.35-44.
Margata, L., Silalahi, J., Harahap, U. and Satria, D., 2018.
The effect of dietary oils and hydrolyzed coconut oil
on minerals absorption in rats. Asian Journal of
Pharmaceutical and Clinical Research, 11(1), p.185-
190.
McKee, T. and McKee, J.R., 2003. Biochemistry: The
Molecular Basis of Life, McGraw-Hill. New York, 3
rd
edition.
Mechanick, J.I., 2006. The rational use of dietary
supplements, nutraceuticals, and functional foods for
the diabetic and prediabetic patients. In: Mechanick
and Brett, editors. Nutritional Strategies for the
Diabetic and Prediabetic Patient, Taylor & Francis,
CRC. New York, p.265-296.
Mohammed, A. and Luka, C.D., 2013. Effect of coconut
oil, coconut water and palm kernel oil on some
biochemical parameters in albino rats. Journal of
Pharmacy and Biological Sciences, 6(3), pp.56-59.
Ng, Z., Chua, K., Iqbal, T. And Kuppusamy, U., 2013.
Soluble receptor for advanced glycation end-product
(sRAGE)/pentosidine ratio: a potential risk factor
determinant for type 2 diabetic retinopathy.
International Journal of Molecular Sciences, 14(4),
pp.7480-7491.
Paliyath, G., Bakovic, M. and Shetty, K., 2011. Functional
Foods, Nutraceuticals and Degenerative Disease
Prevention, Wiley-Blackwell. UK.
Pujol, J., Christinat, N., Ratinaud, Y., Savoia, C., Mitchel,
S. and Dioum, E., 2018. Coordination of gpr40 and
ketogenesis signaling by medium chain fatty acids
regulates beta cell function. Nutrients, 10(4), p.473.
Rayfield, E.J. and Valentine, M.V., 2006. Pathophysiology
and clinical management of diabetes and prediabetes.
In: Mechanick and Brett, editors. Nutritional
Strategies for the Diabetic and Prediabetic Patient,
Taylor & Francis, CRC. New York, p.16-44.
Siddalingaswamy, M., Rayaorth, A. and Khanum, F.,
2011. Anti-diabetic effects of cold and hot extracted
virgin coconut oil. Journal of Diabetes Mellitus, 1(4),
pp.118-123.
Silalahi, J., Rosida, R., Putra, E.D.L. and Satria, D., 2016.
Hypoglycemic effect of hydrolyzed palm kernel oil in
rats. Der Pharma Chemica, 8(20), p.182-186.
Supriatna, D., Uray, A.D., Astawan, M., Muchtadi, D.
And Wresdiyati, T., 2018. The effect of VCO
processing method on blood glucose, cholesterol and
pancreatic profile of diabetic mellitus rats (Sprague
Dawley). Warta IHP, 35(2), p.91-98.
Szkudelski, T., 2001. The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas.
Physiological Research, 50(6), pp.537-546.
Triplitt, C.L., Reasner, C.A. and Isley, W.L., 2005.
Diabetes mellitus. In: DiPiro, J.T., Talbert, R.L., Yee,
G.C., Wells, B.G. and Posey, L.M. Pharmacotherapy:
A Patophysiologic Approach, The McGraw-Hill
Companies, Inc. USA, 6
th
edition.
Vala, G.S. and Kapadiya, P.K., 2014. Medicinal benefits
of coconut oil. International Journal of Life Sciences
Research, 2(4), pp.124-126.
Vassalotti, J.A., 2006. Nutritional strategies for the patient
with diabetic nephropathy. In: Mechanick and Brett,
editors. Nutritional Strategies for the Diabetic and
Prediabetic Patient, Taylor & Francis, CRC. New
York, p.149-169.
Yin, D., Tao, J., Lee, D.D., Shen, J., Hara, M., Lopez, J.,
Kuznetsov, A., Philipson, L.H. and Chong, A.S., 2006.
Recovery of islet β-cell function in streptozotocin-
induced diabetic mice: an indirect role for the spleen.
Diabetes, 55(12), p.3256-3263.
Wong, F.N., Chua, K.H., Tan, J.A.M.A., Wong, C.M. and
Kuppusamy, U.R., 2018. Glycaemic control in type 2
diabetic patients with chronic kidney disease: the
impacts on enzymatic antioxidants and soluble RAGE.
PeerJ, 6, p.e4421.
The Antidiabetic and Antioxidant Activities of Hydrolyzed Virgin Coconut Oil in Streptozotocin-induced Diabetic Rats
159
