
Correlation between Adiponectin Receptor (AdipoR) with
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR),
Peroxisome Proliferator-Activated Receptor Gamma (PPAR-ϒ), and
p38-mitogen-activated protein kinases (MAPK) in
Type 2 Diabetic Rats Treated with Puguntano
(Turanga feel-terrae Lour.) Leaves Extract
Dharma Lindarto
1*
, Melati Silvani Nasution
2
, Santi Syafril
1
, Awaluddin Saragih
1
1
Department of Internal Medicine, Faculty of Medicine, Universitas Sumatera Utara, H. Adam Malik General Hospital,
Medan, Indonesia;
2
Department of Pharmacology and Therapeutic, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia.
Keywords: T2DM, HOMA-IR, PPAR-ϒ, p38-MAPK, Puguntano (Curanga fel-terrae Lour.)
Abstract: Adiponectin has a role in regulating metabolic processes, anti-inflammation, and insulin sensitizers. This
study aimed to examine the correlation between adiponectin receptor (AdipoR) with FPG, insulin, HOMA-
IR, PPAR-ϒ, and p38MAPK in rats model of T2DM. A total of 48 Wistar rats aged 8-10 weeks with a
weight of 180-200 grams were selected. The Wistar rats were induced into T2DM by feeding high-fat
(HFD) diet and injecting small doses of streptozotocin (STZ) 30 mg/kg.bw. The Wistar rats were then
divided randomly into 2 groups: control group (n=24) and treatment group (n=24). The treatment group was
given puguntano leaves extract at a dose of 200 mg/kg. BW once daily for 10 days. After that, AdipoR,
FPG, insulin, HOMA-IR, PPAR-ϒ, and p38MAPK levels were examined. FPG, insulin, and HOMA-IR
levels were significantly lower, but p38MAPK and AdipoR levels were significantly higher in the treatment
group than in the control group. In the group of all subjects, there was a significant correlation between
AdipoR with FPG, insulin, HOMA-IR, PPAR-ϒ and p38MAPK (r=-0.536, p=0.000**, r=-
0.416, p=0.003**; r=-0.478, p=0.001**; r=0.587, p=0.000**; respectively). There was a significant
correlation between AdipoR with the characteristic of insulin resistance (HOMA-IR) and pathways of post-
receptor insulin (PPAR-ϒ and p38MAPK).
1 INTRODUCTION
Adiponectin is widely known as an insulin sensitizer
based on its identification, characterization, and
underlying mechanisms. Adiponectin can reduce
plasma glucose level of rats by suppressing liver
glucose production or increasing glucose uptake in
the peripheral which is independent of insulin (Berg
et al, 2001) inhibiting phosphoenolpyruvate
carboxykinase, glucose-6-phosphatase expression
(Yamauchi et al, 2002), 5'-AMP-activated protein
kinase (AMPK), thereby improving insulin
resistance and preventing hepatosteatosis (Liu et al,
2012). Based on other studies, giving adiponectin
will increase fatty acid oxidation in the skeletal
muscle thereby reducing the content of triglycerides
in muscles and liver and increasing insulin
sensitivity in vivo (Yamauchi et al, 2001).
Adiponectin suppresses the inflammatory response
to macrophages in the tissue (Iannitti et al, 2015).
The effect of adiponectin is mediated by
adiponectin receptors namely AdipoR1 and
AdipoR2 by regulating the expression of metabolic
genes and insulin sensitivity in the target tissue of
insulin (Yamauchi et al, 2007). The expression of
both adiponectin receptors increases fatty acid
oxidation, decreases liver triglyceride levels,
improves insulin resistance, modulates food intake
and energy expenditure, and reduces inflammation.
There is a correlation between mRNA expression
from adiponectin receptors and adiponectin
(Yamauchi et al, 2007; Yamauchi and Kadowaki,
52
Lindarto, D., Nasution, M., Syafril, S. and Saragih, A.
Correlation between Adiponectin Receptor (AdipoR) with Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Peroxisome Proliferator-Activated Receptor Gamma (PPAR-),
and p38-mitogen-activated protein kinases (MAPK) in Type 2 Diabetic Rats Treated with Puguntano (Turanga feel-terrae Lour.) Leaves Extract.
DOI: 10.5220/0009855900520055
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 52-55
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2008). According to previous studies, administration
of the ethanol extract of puguntano leaves (C. feel-
terrae [Lour.]) can significantly improve glucose
metabolism and insulin resistance and increase
adiponectin (Lindarto et al, 2016) and adiponectin
receptor (Lindarto et al, 2019), p38 mitogen-
activated protein kinase (MAPK) levels and GLUT-
4 expression (Syafril et al, 2019) in the treatment
group than in the control group.
The aim of this study was to investigate the
correlation between adiponectin receptor with
fasting plasma glucose (FPG), insulin, homeostatic
model assessment for insulin resistance (HOMA-
IR), peroxisome proliferator-activated receptor
gamma (PPAR-ϒ), and p38 MAPK in T2DM Wistar
rats treated with puguntano leaves extract.
2 METHODS
The study used 48 male Wistar rats aged 8-10 weeks
with a weight of 180-200 grams. Rats were placed
under the natural light cycle at a temperature of 22-
25°C. The Wistar rats were induced T2DM by
feeding HFD for 5 weeks, followed by
intraperitoneal STZ injections 30 mg/kg.BW (
Sigma-Aldrich, Munich, Germany). FPG was
measured from the blood of the lateral tail vein using
a glucometer in which FPG > 200 mg/dL was
considered T2DM (Zhang et al, 2008). Rats were
divided randomly into two groups (control group,
n=24, and treatment group, n=24). The treatment
group was given ethanol extract of puguntano leaves
200 mg/kg.bw/day for 10 days using an orogastric
cannula. The control group was sacrificed when was
diagnosed with T2DM, while the treatment group
was sacrificed after 10 days of treatment. The
sacrifice was done by administering anesthesia
(ketamine), and the head of the rats was beheaded.
After the blood was collected from the left ventricle,
FPG (spectrophotometry) and fasting insulin
(sandwich ELISA) were examined.
Skeletal muscle samples were processed by
homogenization in a cold homogenizing buffer (-
80°C) which was used to determine the level of p38-
mitogen-activated protein kinases (MAPK), PPAR-
ϒ, and AdipoR with Qayeebio kit (China).
The research was conducted at the Molecular
Genetics Laboratory, Faculty of Medicine,
Universitas Padjadjaran Bandung. The ethanol
extract of puguntano leaves was obtained by
maceration method in the Department of Biological
Pharmacy, Faculty of Pharmacy, Universitas
Sumatera Utara, Medan, Indonesia (Kemenkes RI,
2013). This research has been approved by the
Ethics Committee of the Universitas Sumatera
Utara, Medan, Indonesia (Reference 42 / TGL /
KPEK FK USU-RSUP HAM / 2018).
Statistical analysis was performed using SPSS
22.0 software. All data are expressed as a
meanstandard deviation. The Wilcoxon test was
used to compare non-normally distributed groups,
while the Pearson’s or Spearman’s test was used for
the correlation test. A p-value < 0.05 indicated a
statistically significant difference.
3 RESULTS
In Table 1, the FPG, Insulin, HOMA-IR, PPAR, and
p38-MAPK levels in the control group had a
significant difference with the treatment group.
Table 1. Characteristic Baselines of Subjects
Characteristic
Group
p
a
All subjects (n=48)
MeanSD
Control (n=24)
(MeanSD)
Treatment (n=24)
(MeanSD)
FPG (mg/dl)
256.10153.68 375.58
29.15 136.63
33.62
0.000**
Insulin
54.645.58 57.36
6.28 52.32
3.32
0.001**
HOMA-IR
1.951.17 3.05
0.51 0.86
0.20
0.000**
PPAR-ϒ(ng/mL)
35.187.46 29.56
1.06 40.80
6.83
0.000**
p38-MAPK (ng/mL)
22.253.82 20.81
3.02 23.70
4.04
0.005**
AdipoR (ng/mL)
15.223.21 13.79
1.47 16.64
3.83
0.000**
Data are expressed as a mean standard deviation; Wilcoxon test.
a
: Control Group vs Treatment Group;
FPG: fasting plasma glucose; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; PPAR-ϒ;
Peroxisome Proliferator-Activated Receptor-ϒ; p38-MAPK: p38 mitogen-activated protein kinase.
*: < 0.05; **: <0.01.
Correlation between Adiponectin Receptor (AdipoR) with Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Peroxisome
Proliferator-Activated Receptor Gamma (PPAR-), and p38-mitogen-activated protein kinases (MAPK) in Type 2 Diabetic Rats Treated with
Puguntano (Turanga feel-terrae Lour.) Leaves Extract
53
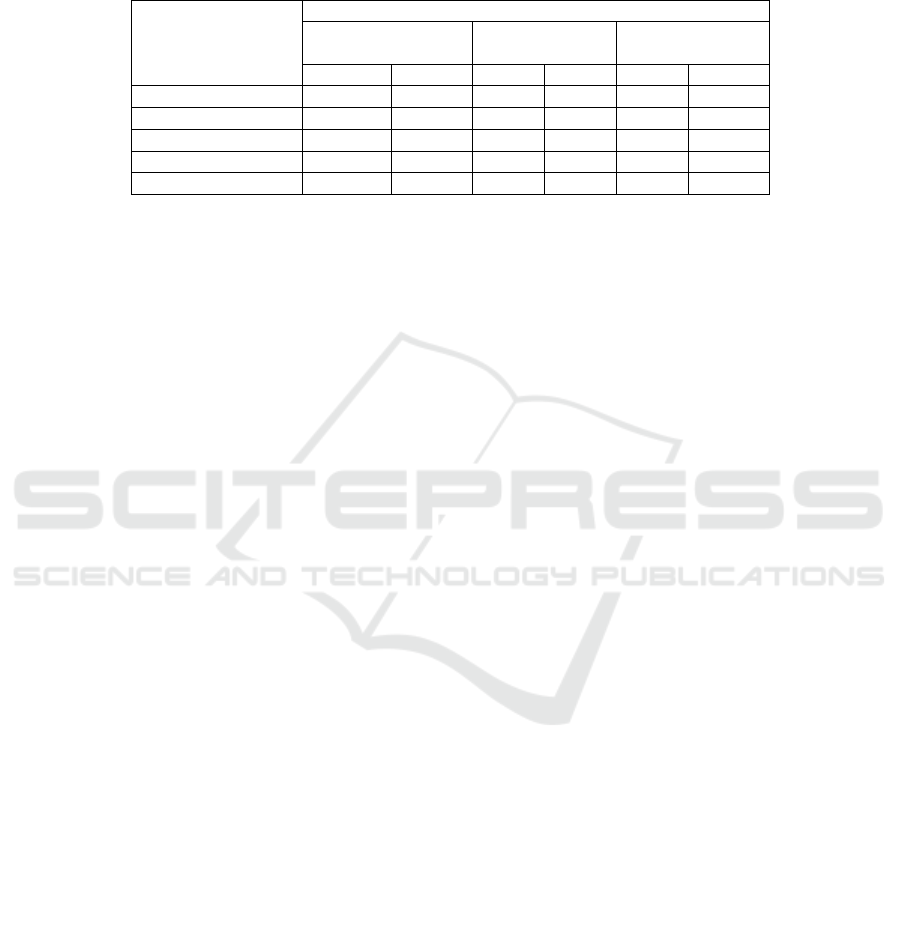
Table 2. shows that AdipoR in all subject group
significantly correlated with insulin, HOMA-IR, and
PPAR-ϒ, whereas there was no correlation found in
the control and treatment groups, except for p38-
MAPK.
Table 2. Correlation between AdipoR with HOMA-IR, PPAR-ϒ, and p38-MAPK in all groups
Characteristic
Group
All subjects (n=48) Control
(n=24)
Treatment
(n=24)
r p r p r p
FPG (mg/dl) -0.536 0.000** -0.274 0.196 -0.264 0.212
Insulin -0.416 0.003** 0.024 0.912 -0.402 0.052
HOMA-IR -0.478 0.001** -0.033 0.878 -0.254 0.231
PPAR-ϒ (ng/mL) 0.587 0.000** 0.088 0.680 0.578 0.003**
p38-MAPK (ng/mL) -0.063 0.670 -0.334 0.106 -0.273 0.197
Data are expressed as mean standard deviation; Wilcoxon test.
a
: Group 1 vs Group 2; FPG: fasting
plasma glucose; HOMA-IR: Homeostatic Model Assessment for Insulin Resistance; PPAR-ϒ;
Peroxisome Proliferator Activated Receptor-ϒ; p38-MAPK: p38-mitogen-activated protein kinase.
*: < 0.05; **: <0.01.
4 DISCUSSION
Secondary metabolites of the ethanol extract of
puguntano leaves identified were glycosides (Zhou,
2005), flavonoids (Huang, 1998), saponins (Fang et
al, 2009), and terpenoids (Wang et al, 2006), which
can decrease blood sugar levels by stimulating the
production and secretion of insulin. Tannin increases
glucose uptake via insulin signaling pathways such
as phosphoinositide 3-kinase (PI3K), p38-MAPK,
and GLUT-4 translocation (Kumari and Tannins,
2012). AdipoR improves glucose metabolism
through mechanisms such as increases fatty acid
oxidation in the muscles and suppresses lipid
cumulation in the liver by activating AMPK so that
the content of triglycerides in the liver and muscles
decreases and insulin sensitivity is improved
(Yamauchi et al,2001).
Adiponectin can stimulate the improvement of
the p38-MAPK pathway (Mao et al, 2006) as an
anti-inflammatory effect (Xin et al, 2011) and the
enhancement of PPAR-ϒ expression through
increasing 3T3-L1 cells associated with
differentiation of adipocytes (Fu et al, 2005).
Adiponectin in diabetic patients had a significant
negative correlation with BMI and positive
correlation with systolic blood pressure and
microalbuminuria (El Dayem et al, 2015).
In this study, treatment with puguntano
significantly improved FPG, insulin, HOMA-IR,
PPAR-ϒ, p38-MAPK, and AdipoR. The increase in
adiponectin receptor correlates significantly with
insulin, HOMA-IR, and PPAR-ϒ in all subjects
groups.
Conclusion: Puguntano treatment reduces the
risk of cardiovascular diseases, while an increase in
adiponectin receptor is associated with improved
insulin resistance (HOMA-IR) and post-receptor
insulin (PPAR-ϒ and p38-MAPK). In addition to
insulin sensitivity and post-receptor insulin,
adiponectin and AdipoR has various working
mechanisms.
ACKNOWLEDGMENT
The authors acknowledge the assistance of the
Molecular Genetics Laboratory, Faculty of
Medicine, Universitas Padjajaran, Bandung.
CONFLICT OF INTEREST
The author stated that there is no conflict of interest
regarding the publication of this article
REFERENCES
Berg AH, Combs TP, Du X, Brownlee M, Scherer PE,
2001. The adipocyte-secreted protein Acrp30
enhances hepatic insulin action. Nat. Med. 7(8):947-
53.
El Dayem SMA, Nazif HK, EI-Kader MA, El-Tawil M.
Study of Adiponectin Level in Diabetic Adolescent
Girls in Relation to Glycemic Control and
Complication of Diabetes. OA Maced J Med Sci.
2015;3(4):613-8.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
54

Fang H, Ning DS, dan Liang XY, 2009. Studies on
Technology Optimization for Extracting Triterpenoid
Saponins from Picria felterrae by Multi-Target
Grading Method. Journal of Chinese Medicinal
Material. 32(12):1902-5.
Fu Y, Luo N, Klein RL, Garvey WT, 2005. Adiponectin
promotes adipocyte differentiation, insulin sensitivity,
and lipid accumulation. J. Lipid Res. 46;1369–79.
Huang Y, de Bruyne T, Apers S, Ma Y, Claeys M, van
den Berghe D, Pieters L, Vlietinck, A, 1998.
Complement-Inhibiting Cucurbitacin Glycosides from
Picria felterrae. Journal of Natural Products.
61(6):757-61.
Iannitti T, Graham A, Dolan S, 2015. Adiponectin-
mediated analgesia and anti-inflammatory effects in
the rat. PLoS One. 10(9):e0136819.
Kemenkes RI. Farmakope Herbal Indonesia, 2013. Ed.I
Suplemen II. Kemenkes RI Jakarta.106-7.
Kumari M and Tannins JS, 2012. An Antinutrient with
Positive Effect to Manage Diabetes Res. J. Recent Sci.
1(12):1-8.
Lindarto D, Machrina Y, Syafril S, Saragih A, 2019. The
Effect Of Puguntano (Curanga Fel-Terrae [Lour.])
Extract On Adiponectin Receptor (Adipor) In Rats
With Type 2 Diabetes Mellitus. Asian J Pharm Clin
Res, 12(2):551-3.
Lindarto D, Syafril S, Zein U, Saragih A, 2016. The
Effect Of Dhawalsan-1 (Curanga Fel-Terrae [Lour.])
Extract Versus Metformin On The Metabolic And
Inflammatory Characteristics Of Patients With Newly
Diagnosed Type 2 Diabetes Mellitus. Asian J Pharm
Clin Res. 9 Suppl 1:225-8.
Liu M, Xiang R, Wilk SA., Zhang N, Sloane
LB, Azarnoush K, et al, 2012. Fat-specific Dsb A-L
overexpression promotes adiponectin multimerization
and protects mice from diet-induced obesity and
insulin resistance. Diabetes. 61(11):2776-86.
Mao X, Kikani, CK, Riojas RA, Langlais P, Wang
L, Ramos FJ, et al, 2006. APPL1 binds to adiponectin
receptors and mediates adiponectin signaling and
function. Nat. Cell Biol. 8(5):516–23.
Syafril S, Lindarto D, Lelo A, Sembiring RJ, Manaf A,
Putra IB, Hasibuan PAZ, Mutiara E, 2019. The Effect
of Puguntano Leaf Extract (Curanga Fel Terrae
Merr.) on p38-MAPK Levels and Glut-4 Expression
in Type 2 Diabetic Rat Muscle. OA Maced J Med
Sci. 7(4):521-5.
Wang LS, Li SH, Zou JM, Guo YJ, Sun HD, 2006. Two
New Terpenoids from Picria feel-terrae. Journal of
Asian Natural Product Research. 8(6):491-4.
Xin X, Zhou L, Reyes CM. Liu F, Dong LQ, 2011.
APPL1 mediates adiponectin stimulated p38 MAPK
activation by scaffolding the TAK1-MKK3-p38
MAPK pathway. Am. J. Physiol. Endocrinol. Metab.
2011;300(1):E103–E10.
Yamauchi T, Kadowaki T, 2008. Physiological and
pathophysiological roles of adiponectin and
adiponectin receptors in the integrated regulation of
metabolic and cardiovascular diseases. Int J Obes
(Lond). 32 Suppl 7: S13-8.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki
H, Uchida S, et al, 2002. Adiponectin stimulates
glucose utilization and fatty-acid oxidation by
activating AMP-activated protein kinase. Nat. Med.
8(11):1288-95.
Yamauchi T, Kamon J, Waki H., Terauchi Y, Kubota
N, Hara, et al, 2001. The fat-derived hormone
adiponectin reverses insulin resistance associated with
both lipoatrophy and obesity. Nat. Med. 7(8):941-6.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa
T, Iwabu M, et al, 2007. Targeted disruption of
AdipoR1 and AdipoR2 causes abrogation of
adiponectin binding and metabolic actions. Nat. Med.
13(3):332-9.
Zhang M, Lv XY, Li J, Xu ZG, Chen L, 2008. The
Characterization of a high-fat diet and multiple low-
dose streptozotocin-induced type 2 diabetes rat model.
Exp Diabetes Res.1-9.
Zhou JM, Wang LS, Niu XM, Sun HD, Guo YJ, 2005.
Phenylethanoid Glycosides from Picria felterrae Lour.
Journal of Integrative Plant Biology. 47(5):632-6.
Correlation between Adiponectin Receptor (AdipoR) with Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Peroxisome
Proliferator-Activated Receptor Gamma (PPAR-), and p38-mitogen-activated protein kinases (MAPK) in Type 2 Diabetic Rats Treated with
Puguntano (Turanga feel-terrae Lour.) Leaves Extract
55
