
Effect of Lifestyle Modification Combined with Metformin on Serum
Chemerin Concentration in Metabolic Syndrome Subjects
Brama Ihsan Sazli
1
, Dharma Lindarto
1*
, Dian Anindita Lubis
1
, Melati Silvani Nasution
1
1
Department of Internal Medicine, Faculty of Medicine, Universitas Sumatera Utara
H. Adam Malik Hospital, Jalan Bunga Lau No 17, Medan 20136, Indonesia
Keywords: Lifestyle modification, Chemerin, Metabolic Syndrome.
Abstract: Chemerin is an adipokine that plays an important role in inflammation development and insulin resistance
by accommodating macrophage infiltration into adipose tissue. This study aimed to determine the effect of
lifestyle modification with and without metformin on chemerin in metabolic syndrome. Forty-five metabolic
syndrome subjects (IDF-2005) were randomly assigned to one of the two groups: placebo group (n=22) and
metformin group (n=23). Both groups underwent a 12-week lifestyle modification (diet and moderate
aerobic-exercise). Only 40 participants (placebo group n=20 and metformin group n=20) completed the
survey whereas 5 participants dropped out of the study. After their lifestyle was modified, body weight,
body mass index, waist circumference, and chemerin decreased significantly (P<0.001) in both groups.
Moreover, there was a significant difference between both groups in body weight, BMI, and WC (P<0.05)
but not for chemerin. Thus, lifestyle modification with metformin improved BW, BMI, and WC on
metabolic syndrome, and there were no significant differences in reduced chemerin between placebo and
metformin groups. Further investigations should be done to establish the effect of lifestyle modification
combined with metformin on chemerin after an extended follow-up period.
1 INTRODUCTION
Metabolic syndrome represents a combination
of cardiometabolic risk factors including glucose
intolerance, insulin resistance, central adiposity,
hypertension, non-alcoholic fatty liver disease, and
dyslipidemia. The prevalence of metabolic
syndrome increases rapidly worldwide as a result of
the continuous obesity epidemic.
This will also
increase global risk in the incidence of cardiovascular
disease and type 2 diabetes mellitus (T2DM)
(Bruce and
Byrne, 2009). Adiposity has been known as an
important endocrine organ that does not only store
energy but also regulates metabolism and energy
homeostasis (Rosen,2006)
Chemerin, an adipokine that was recently
found, increased its expression in obesity state
(Goralski et al., 2007; Bozauglu et al., 2007).
Several specific functions of chemerin are regulation
of specific immune cell migration
(Zabel, Silverio
and Butcher, 2007), anti-inflammatory effects on
macrophages (Cash et al., 2008), and regulation of
adipogenesis (Zabel, Silverio, and Butcher, 2007),
Previously, a significant association has been
identified between characteristics of the metabolic
syndrome and circulating chemerin levels in a
relatively small sample of human subjects from
Mauritius (Bozauglu et al., 2007). Bozaoglu et al
(2009) evaluated plasma chemerin concentration in
human subjects and found that plasma chemerin
concentrations were highly associated with body
mass index (BMI), blood pressure, and plasma
triglycerides. And in women with polycystic ovary
syndrome, treatment with metformin decreases
serum chemerin levels. (Tan et al., 2009).
Physical inactivity is well known as the risk
factor for T2DM (Venables and Jeukendrup, 2009)
and aerobic training in obese adults has been shown
to reduce adiposity and insulin resistance (O'Leary et
al., 2006). There has been no previous report about
lifestyle modification induces alteration in chemerin
concentrations in metabolic syndrome, which may
serve a connection between obesity and insulin
resistance. Modification of lifestyle against
overweight, physical inactivity, and atherogenic diet
has been recommended as a primary in the
management of metabolic syndrome (Eckel, Grundy
and Zimmet, 2005). However, lifestyle
modifications alone often cannot achieve clinically
38
Sazli, B., Lindarto, D., Lubis, D. and Nasution, M.
Effect of Lifestyle Modification Combined with Metformin on Serum Chemerin Concentration in Metabolic Syndrome Subjects.
DOI: 10.5220/0009855700380043
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 38-43
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

meaningful weight loss (Miler, Kojeca and
Hamilton, 1997).
Metformin, a biguanide insulin sensitizer agent
has been shown to decrease body weight,
hyperinsulinemia, and hyperglycemia in adult
patients with T2DM (UKPDS 34, 1998). Metformin
has recently also been suggested to increase the
effect of insulin sensitivity from exercise (Sharoff et
al., 2010), inhibit platelet aggregation, as an
antioxidant activity, reduce weight, and gives effect
on lipid parameters like total cholesterol, low-
density lipoprotein cholesterol (LDL-C),
triglycerides (TG), high-density lipoprotein
cholesterol (HDL-C), and arterial hypertension
[Glueck et al., 2001; Wulffele et al., 2004).
Metformin does not induce hypoglycemia so it can
be given safely to euglycemic patients (Pasquali et
al.,200)
Therefore, the aim of this study was to
evaluate the effect of lifestyle modification
combined with metformin on chemerin in metabolic
syndrome.
2 MATERIALS AND METHODS
The research subjects were recruited in Haji Adam
Malik Hospital, Medan, Indonesia. From 184 obese
nurses (BMI ≥25), only 45 subjects were diagnosed
with metabolic syndrome (IDF-2005) and agreed to
involve in lifestyle modification for 12 weeks.
Diagnosis of metabolic syndrome by IDF-2005
guidelines were used, namely central obesity with a
Waist Circumference (WC) of ≥90 cm for men and
≥80 cm for women (Asian population), and any two
of the following four factors: (1) triglyceride ≥150
mg/dl or specific treatment for lipid abnormality; (2)
HDL-C (men <40 mg/dl and women <50 mg/dl) or
specific treatment for lipid abnormality; (3)
hypertension ≥130/85 mmHg or history of treatment
previously diagnosed hypertension; and (4) FBS
≥100 mg/dl or previously diagnosed T2DM (WHO,
2004: Grundy et al., 2005). They were divided
randomly to either the placebo group (n=22) or
metformin group (n=23).
All subjects gave their fully informed consent
before participated in the study. The Ethics
Committee of Medical Faculty of Universitas
Sumatera Utara has approved the study. We
excluded subjects who had cardiovascular diseases
or any other major illnesses, smokers, or were taking
medications that could affect the laboratory test
results. Before and during the study, trained health
nurses and participants discussed the lifestyle
modification programs including diet and exercise.
To facilitate changes in behavior, each participant
receives leaflets and diaries to record their
behavioral performance, diet, physical activity, WC,
and body weight (BW) monitored by phone
. Participants attended a follow-up meeting every
week to confirm how the participants had complied
with the targeted behaviors and checked whether the
participants had experienced any health and safety
problems related to behavioral changes including the
side effect of drugs.
2.1 Anthropometric Measurements
Weight in kilograms (kg) and heights in meters (m)
were measured, and the weight in kilograms divided
by the square of the height in meters to calculated
BMI as For the Asian population, BMI <18.5 is
classified as underweight, BMI 18.5-22.9 is
classified as normal, BMI 23-24.9 is classified as
overweight, BMI 25-29.9 is classified as obese I and
BMI ≥30.0 is classified as obese II. The WC was
measured midway between the uppermost border of
the iliac crest and the lower border of the coastal
margin (rib cage), and using Asian values (male ≥90
cm; female ≥80 cm) (WHO, 2004). The exercise
program consists of moderate aerobic exercise at
least 3 times per week (30 minutes each) (Misra,
Misra and Wijesuriya, 2006). The aerobic exercise
was supervised by a physiotherapist at each training
session. The exercise group performed a warm-up
exercise for 5 min, followed by the main exercise for
20 min, and relaxation exercise for 5 min at the end
of the exercise period (PERKENI, 2015).
2.2 Diet
Between 0 and 12-week period throughout the study,
all subjects followed a standard weight maintenance
diet (55–60% carbohydrate, 15–20% protein, and
20–25% fat) (PERKENI, 2015). All subjects were
free to consume and choose the food according to
their dietary habits and from the list of food
replacement.
2.3 Blood Pressure and Blood Sample
Analysis
Blood pressure was measured by an average of twice
measurement after a 10-minute break with a mercury
sphygmomanometer. After overnight fasting and
collection, blood samples were centrifuged for 15
min while plasma and serum containing tubes were
stored at - 20
0
C until analysis. Blood glucose levels
Effect of Lifestyle Modification Combined with Metformin on Serum Chemerin Concentration in Metabolic Syndrome Subjects
39
were measured by photometer autoanalyzer Modular
P 800, plasma HDL-C and LDL-C were measured
by the Architect Ci 8200 (Abbott, USA), triglyceride
was measured by GPO-PAP methods of Architect,
hs-CRP was measured by sensitive immunoassay
(Siemens Medical Solution Inc, IL, USA) of
Immulite 1000, HbA1c was measured by high-
performance liquid chromatographic (HPLC) of the
Bio-Rad D 10, and chemerin was measured by
Mediagnost ELISA E-102 (Sandwich-Assay).
2.4 Statistical Analysis
Data were presented as mean ± SD. The normality
assumption of the placebo group and metformin
group data were evaluated and confirmed using
Shapiro-Wilk in each group. Differences between
and within each data of the placebo group and
metformin group were tested using an independent
sample t-test and dependent t-test. However, the
abnormal data were tested using the Mann-Whitney
U test and Wilcoxon test. Two-sided P-values of
<0.05 were considered as statistically significant.
The data were analyzed using SPSS 22 software.
3 RESULTS
Of the 45 participants at the baseline, 40 participants
(placebo group, n=20; metformin group, n=20)
completed in the 12-week survey, whereas 5
participants (2 participants from the placebo group
and 3 participants from the metformin group)
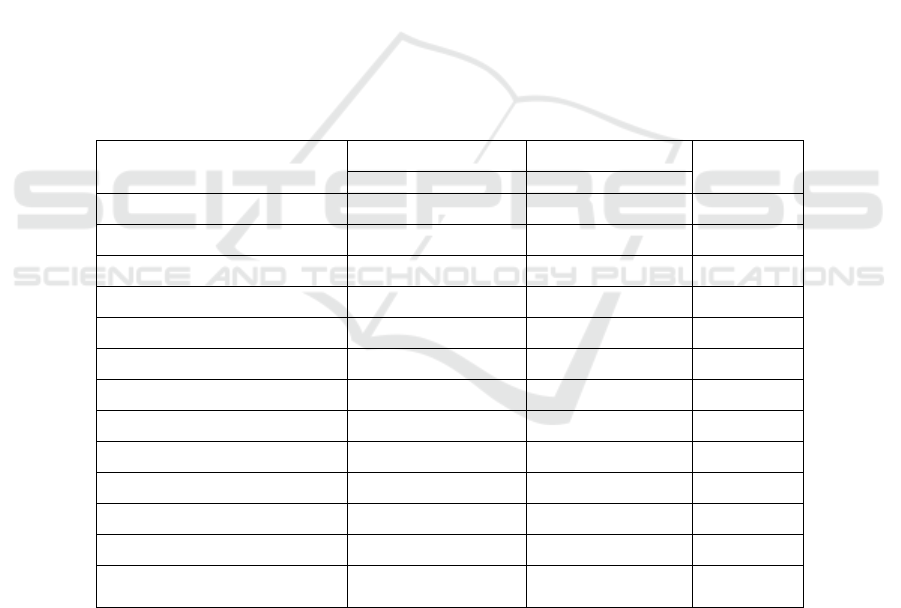
dropped out of the study. In Table 1, there was no
significant difference in the baseline characteristics
of the two groups.
In Table 2, after 12 weeks of lifestyle
modification, there was a significant decrease in
BW, BMI, WC, SBP, and chemerin in placebo and
metformin group. But there were no statistically
significant differences in reduced chemerin between
the two groups.
Table 1: Clinical Characteristics of Placebo and Metformin Groups on Metabolic Syndrome Subjects
Variable Placebo group Metformin group P
a
=22 =23
n (F/M) 17/5 20/3
Age (yr) 40.41±5.61 42.91±5.62 0.142
BW (kg) 76.98±11.64 80.53±14.72 0.374
BMI (kg/m
2
) 32.11±4.09 34.03±5.76 0.206
WC (cm) 95.40±7.41 97.23±10.95 0.927
SBP (mmHg) 123.18±11.29 125.21±19.74 0.567
DBP (mmHg) 82.00±9.99 80.65±10.69 0.247
HDL-C (mg/dl) 45.72±8.48 48.34±15.47 0.918
TG (mg/dl) 150.40±51.05 152.00±63.87 0.974
FBG (mg/dl) 85.27±11.98 86.69±10,06 0.351
PPG (mg/dl) 115.72±11.98 104.21±21.40 0.401
hs-CRP (mg/dl) 3.45±2.47 3.83±2.34 0.467
Chemerin (ng/mL) 344.09±104.58 345.15±83.90 0.970
BW: body weight; BMI: Body Mass Index; CRP: C-reactive protein; DBP: Diastolic Blood
Pressure: FBS: fasting Blood Sugar; PPG: Postprandial Glucose; SBP: Systolic Blood Pressure;
TG: Triglyceride; WC: Waist Circumference; PPG: Postprandial glucose.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
40
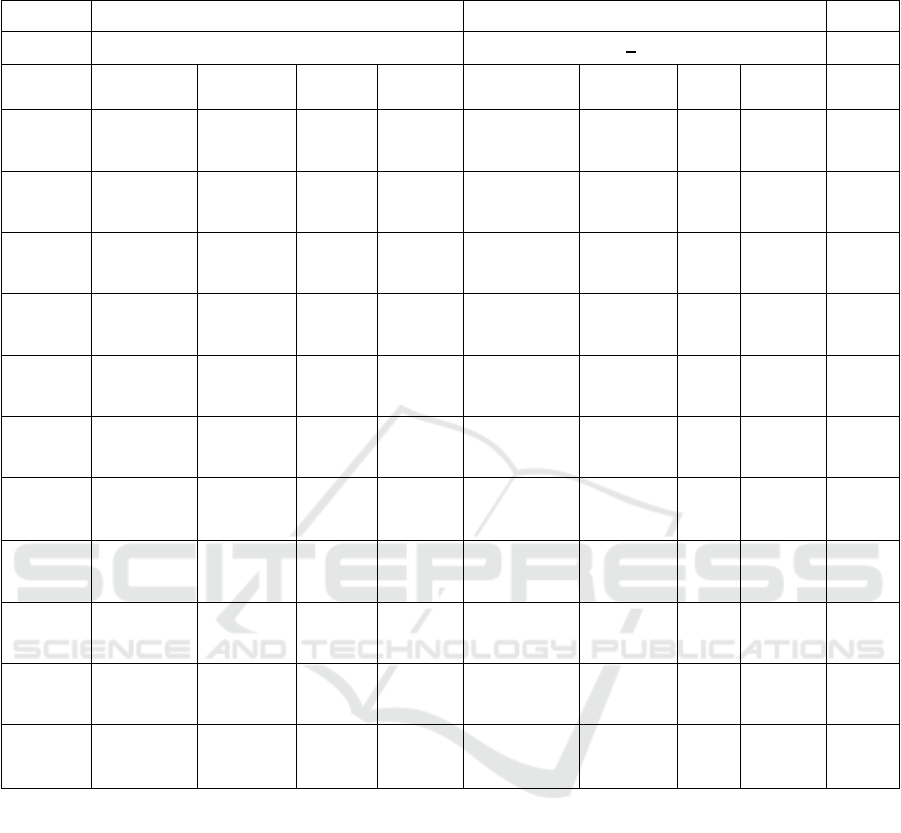
Table 2: Clinical Characteristics of Placebo and Metformin Groups after 12-Week Follow-Up for both Groups of Metabolic
Syndrome Subjects
Placebo group Metformin group
Variable (n=20) (n=20) P
d
Baseline At 12-
week
Differe
nce
P
b
Baseline At 12-
week
Diffe
rence
P
c
Weight
(kg)
77.6 ± 11.0 75.2±10.8 -2.3 0.001** 81.4±14.6 77.4±14.5 -3.9 0.001** 0.001*
*
BMI
(kg/m
2
)
32.1 ± 4.1 30.9±4.1 -1.1 0.001** 34.2±5.6 32.4±5.6 -1.8 0.001** 0.002*
*
WC
(cm)
95.7 ± 7.3 89.9±7.5 -5.8 0.001** 97.9±11.5 91.8±10.7 -6.2 0.001** 0.047*
SBP
(mmHg)
123.5±11.4 114.0±8.2 -9.5 0.007** 127.0±20.3 112.8±8.5 -14.3 0.001** 0.231
DBP
(mmHg)
82.2±10.5 69.0±5.5 -31.8 0.001* 80.6±11.0 67.5±7.2 -13.3 0.001** 0.300
HDL-C
(mg/dl)
46.4±8.5 45.3±10.0 -1.1 0.628 48.9±16.4 46.5±9.8 -2.4 0.653 0.103
TG
(mg/dl)
147.5±50.5 153.3±67.
9
5.8 0.634 152.3±66.9 149.0±10
2.4
-3.3 0.147 0.285
FBS
(mg/dl)
83.4±10.6 91.7±20.6
4
8.3 0.001* 84.9±8.9 87.7±10.7 2.8 0.305 0.039*
PPG
(mg/dl)
114.9±35.4 112.3±37.
7
-2.5 0.717 105.1±22.4 102.3±19.
3
-2.8 0.491 0.142
CRP
(mg/dl)
3.6±2.5 3.0±2.2 -0.6 0.048* 3.9±2.4 3.5±1.9 -0.6 0.327 0.482
Chemeri
n
(ng/mL)
339.5±106.
6
225.5±43.
7
-113.9 0.001** 339.9±82.9 226.5±46.
1
-
113.9
0.001** 0.430
BMI: Body Mass Index; CRP: C-reactive protein; DBP: Diastolic Blood Pressure: FBS: Fasting Blood Sugar; PPG; SBP:
Systolic Blood Pressure; TG: Triglyceride; WC: Waist Circumference; 2h-PPG: 2 hour-Postprandial glucose.
a
Difference between the baseline and 12-week follow-up surveys
b
Difference between the baseline and 12-week follow-up surveys in the Placebo group, based on a dependent t-test.
c
Difference between the baseline and 12-week follow-up surveys in the Metformin group, based on a dependent t-test.
d
Δ Difference between the Placebo group and Metformin group after the 12-week follow-up surveys, based on an
independent t-test.
*P < 0.05, **P < 0.01
4 DISCUSSIONS
Adipose tissue is an active organ secreting many
metabolically important proteins known as
adipokines (Lau et al., 2006). Some of these
adipokines have important functions in insulin
resistance and cardiovascular complications with
central or visceral obesity (Murakami et al., 2006).
The latest systematic review of the literature
promotes the dose-response effect of aerobic
exercise on visceral adiposity, but the ability of
exercise to reduce visceral adipose tissue is less
significant in those who have metabolic disorders
)Kelley and Kelley, 2007). Thus, it remains unclear
whether a dose-response of exercise on central
Effect of Lifestyle Modification Combined with Metformin on Serum Chemerin Concentration in Metabolic Syndrome Subjects
41

adiposity is also consistent in metabolic syndrome.
Nevertheless, regular exercise plays an important
role in abdominal fat loss during weight
maintenance and can avoid weight gain in those who
have successfully reduce body weight. (Wing and
Hill, 2001). Chemerin may play an important role in
the metabolic syndrome and may be an independent
promising adipokine marker.
In these studies, lifestyle modification
decreased BW, BMI, WC, diastolic BP, and
chemerin significantly in the placebo group and
metformin group, whereas FBS and CRP only
decreased significantly in the placebo group.
Saremi et al in 2010 showed that chemerin
levels decreased significantly after body weight
reduction (particularly visceral fat) in overweight
and obese males after 12-week of aerobic training.
The recent results of a large-scale epidemiological
study from Mauritius also indicate the same results
(Bozaouglu et al., 2007).
Plasma CRP levels are well known to be an
important part of systemic inflammation. Some
previous studies have reported the effect of exercise
training on plasma CRP concentrations. Mattusch et
al. found a significant reduction in CRP levels after
nine months of marathon training in 12 athletes.
Furthermore, Smith et al. also reported lower CRP
levels in 43 volunteers after six months of exercise
training. Based on these studies, chemerin decreased
more significantly than CRP, and the future
chemerin might replace the position of CRP as the
key index of systemic inflammation.
Thus, lifestyle modification with metformin
improved BW, BMI, WC, and chemerin on
metabolic syndrome. But there were no significant
differences in reduced chemerin between placebo
and metformin groups. Esteghamati et al in 2014
found 3 months monotherapy with metformin was
associated with a significant reduction in chemerin
in type 2 diabetes patient, so we need further
evaluation of using metformin to reduce chemerin in
a nondiabetic patient like in this study.
There are some limitations to our study.
Some detailed exercise records by the participant
were not obtained, which can attenuate the outcome
of some adipokines. In addition, we did not evaluate
whether the beneficial effects on b-cell function,
insulin sensitivity, glycemic control, other
inflammatory parameters support this result. We
evaluated only a limited number of inflammation
biomarkers. Longer and larger sample size studies
are needed to evaluate the positive effects lifestyle
and metformin on chemerin level, as to prevent
cardiovascular event related to metabolic syndrome.
5 CONCLUSIONS
Lifestyle modification with metformin improved
BW, BMI, WC on metabolic syndrome, and there
was no significant decrease of chemerin between
placebo and metformin groups. Further
investigations should be done to confirm the effects
of lifestyle modification combined with metformin
on chemerin after an extended follow-up period.
REFERENCES
Bruce KD and Byrne CD. 2009. The metabolic syndrome:
common origins of a multifactorial disorder. Postgrad
Med, 85; pp.614–21.
Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J,
Collier G, et al. 2007. Chemerin is a novel adipokine
associated with obesity and metabolic syndrome.
Endocrinology,148, pp. 4687-94.
Bozaoglu K, Segal D, Shields KA, Cummings N, Curran
JE, Comuzzie AG, et al. 2009. Chemerin is associated
with metabolic syndrome phenotypes in a Mexican-
American population. J Clin Endocrinol Metab, 94,
pp. 3085-8.
Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran
J. 2008. Synthetic chemerin-derived peptides suppress
inflammation through ChemR-23. J Exp Med, 205,
pp.767-75.
Eckel RH, Grundy SM, Zimmet PZ. 2005. The metabolic
syndrome. Diagnosis and Management of the
Metabolic Syndrome. Lancet, 365, pp. 1415-28.
Esteghamati, A., Ghasemiesfe, M., Mousavizadeh,
M., Noshad, S., Nakhjavani, M., 2014.
Pioglitazone and metformin are equally effective
in the reduction of chemerin in patients with type
2 diabetes. J. Diabetes Investig. 5, 327–332
.
Glueck CJ, Fontaine RN, Wang P, Subbiah MT, Weber K,
Illig E, et al. 2001. Metformin reduces weight,
centripetal obesity, insulin, leptin, and low-density
lipoprotein cholesterol in nondiabetic, morbidly obese
subjects with body mass index greater than 30.
Metabolism, 50, pp. 856-61.
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA,
Butcher EC, Parlee SD, et al. 2007. Chemerin, a novel
adipokine that regulates adipogenesis and adipocyte
metabolism. J Biol Chem, 282, pp.28175-88.
Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel
RH, Franklin BA, et al. 2005. Diagnosis and
management of the metabolic syndrome. An American
Heart Association/National Heart, Lung, and Blood
Institute Scientific Statement. Executive summary.
Cardiol Rev, 13, pp.322-7.
Kelley GA, Kelley KS. 2007. Effects of aerobic exercise
on lipids and lipoproteins in adults with type 2
diabetes: a meta-analysis of randomized controlled
trials. Public Health, 121, pp. 643-55.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
42

Lau C, Toft U, Tetens I, Richelsen B, Jorgensen T, Bosch-
Jhansen K, et al. 2006. Association between dietary
glycemic index, glycemic load, and body mass index
in the Inter 99 study: is underreporting a problem? Am
J Clin Nutr, 84, pp.641-5.
Mattusch F, Dufaux B, Heine O, Mertens I, Rost R. 2000.
Reduction of the plasma concentration of C-reactive
protein following nine months of endurance training.
Int J Sports Med, 21, pp.21-4.
Miller WC, Koceja DM, Hamilton EJ. 1997. A meta-
analysis of the past 25 years of weight loss research
using diet, exercise or diet plus exercise intervention.
Int J Obes Relat Metab Disord, 21, pp.941-7.
Misra A, Misra R, Wijesuriya M. 2006. The metabolic
syndrome in South Asians. In: Mohan V, Rao HR,
Gundu HR, editors. Type 2 diabetes in South Asians:
Epidemiology, risk factors, and prevention. New
Delhi, India: Jaypee Brothers, pp.76–96.
Murakami K, Sasaki S, Takahashi Y, Okubo H, Hosoi Y,
Horiguchi H, et al. 2006. Dietary glycemic index and
load in relation to metabolic risk factors in Japanese
female farmers with traditional dietary habits. Am J
Clin Nutr, 83, pp. 1161-9.
O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP,
Gonzalez F, Kirwan JP. 2006. Exercise-induced
reversal of insulin resistance in obese elderly is
associated with reduced visceral fat. J Appl Physiol,
100, pp. 1584-9.
Pasquali R, Gambineri A, Biscotti D, Vicennati V,
Gagliardi L, Colitta, et al. 2000. Effect of long-term
treatment with metformin added to hypocaloric diet on
body composition, fat distribution, and androgen and
insulin levels in abdominally obese women with and
without the polycystic ovary syndrome. J Clin
Endocrinol Metab, 85, pp. 2767-74.
Perkeni. 2015. The consensus of Management and
Prevention of Type 2 Diabetes Mellitus in Indonesia.
Endocrine Association of Indonesia.
Rosen ED, Spiegelman BM. 2006. Adipocytes as
regulators of energy balance and glucose homeostasis.
Nature, 444, pp.847-53.
Saremi A, Shavandi N, Parastesh M, Daneshmand H.
2010. Twelve-Week Aerobic Training Decreases
Chemerin Level and Improves Cardiometabolic Risk
Factors in Overweight and Obesity Men. Asian
Journal of Sports Medicine,1, pp.151-8.
Sharoff CG, Hagobian TA, Malin SK, Chipkin SR, Yu H,
Hirshman MF, et al. 2010. Combining short-term
metformin treatment and one bout of exercise does not
increase insulin action in insulin-resistant individuals.
Am J Physiol Endocrinol Metab, 298, E815–E23.
Smith JK, Dykes R, Douglas JE, Krishnaswamy K, Berk
S. 1999. Long-term exercise and atherogenic activity
of blood mononuclear cells in persons at risk of
developing ischemic heart disease. JAMA. 281, pp.
1722-7.
Tan BK, Chen J, Farhatullah S, Tan BK, Chen J,
Farhatullah S, et al. 2009. Insulin and Metformin
Regulate et al. Insulin and Metformin Regulate
Circulating and Adipose Tissue Chemerin. Diabetes,
58, pp.1971-7.
UKPDS. 1998. Effect of intensive blood-glucose control
with metformin on complications in overweight
patients with type 2 diabetes (UKPDS 34). UK
prospective diabetes study group. Lancet, 352, pp.854-
65.
Venables MC, Jeukendrup AE. 2009. Physical inactivity
and obesity: links with insulin resistance and type 2
diabetes mellitus. Diabetes Metab Res Rev, 25, S18-
23.
WHO Expert Consultation. 2004. Appropriate-body mass
index for Asian populations and its implications for
policy and intervention strategies. Lancet, 363,
pp.157-63.
Wing RR, Hill JO. 2001. Successful weight loss
maintenance. Annu Rev Nutr, 21, :pp. 23-41.
Wulffele MG, Kooy A, de Zeeuw D, Stehouwer,
Gansevoort. 2004. The effect of metformin on blood
pressure, plasma cholesterol, and triglycerides in type
2 diabetes mellitus: a systematic review. J of Internal
Medicine, 256, pp.1-14.
Zabel BA, Silverio AM, Butcher EC. 2005. Chemokine-
like receptor-1 expression and chemerin-directed
chemotaxis distinguish plasmacytoid from myeloid
dendritic cells in human blood. J Immunol, 174,
pp.244-51.
Effect of Lifestyle Modification Combined with Metformin on Serum Chemerin Concentration in Metabolic Syndrome Subjects
43
