
Comparison of Serum MMP-1 Value Levels in Spondylitis
Tuberculose with Degenerative Spine Disease
Alamsyah Faritz Siregar
1*
, Otman Siregar
2
, Nazar Moesbar
3
1
Resident of Orthopaedic and Traumatology, Faculty of Medicine Universitas Sumatera Utara/ Haji Adam
Malik Hospital-Medan
2
Consultant of Orthopaedic and Traumatology, Spine Division, Faculty of Medicine Universitas Sumatera Utara/ Haji
Adam Malik Hospital-Medan
3
Staff of Orthopaedic and Traumatology, Spine Division, Faculty of Medicine UniversitasSumatera Utara/ Haji Adam
Malik Hospital-Medan
Keywords: Matrix metalloproteinase; MMP-1; spondylitis tuberculous; degenerative spine disease.
Abstract: The purpose of this study was to determine differences in serum MMP-1 values in tuberculous spondylitis
with degenerative diseases of the spine. Eighteen (18) subjects were divided into 2 groups, with nine (9)
subjects of spondylitis tuberculose and 9 subjects of degenerative spine disease in the period from December
2017 to November 2018 who were included in the inclusion criteria included in this study and blood sampling
was taken for examination of serum MMP-1 levels. Distribution of samples of spondylitis tuberculous and
degenerative spine diseases with total 18 subjects, with 10 women (55.6%) and men as many as 8 subjects
(44.4%), with the youngest age of the study subject 13 years old and the oldest age is 73 years old research
subjects with mean and standard deviation of 43.72 ± 15.88 years. There were significant differences in serum
MMP-1 levels between spondylitis tuberculous and degenerative spine diseases with a significance value of
0.016 (p<0.05) with mean serum MMP-1 levels in the spondylitis tuberculous study group 1055.56 ± 390.86
and mean in the control group 666.67 ± 250.00. Patients suffering from spondylitis tuberculous have higher
serum MMP-1 levels than patients with degenerative spine disease, although MMP-1 is not a specific marker
examination for spondylitis tuberculous, the results of this study can be suggestive into that can help to
evaluate enzyme activity in patients with spondylitis tuberculous disease.
1 INTRODUCTION
Tuberculosis (TB) is one of the long-known diseases
and is still the leading cause of death in the world. The
prevalence of TB in Indonesia and other developing
countries is quite high. In 2006, new cases in
Indonesia amounted to> 600,000 and most people
suffer from productive age (15–55 years).
About 20% of pulmonary TB infections will
spread out of the lungs (extrapulmonary TB). Eleven
percent of extrapulmonary TB is osteoarticular TB,
and about half of patients suffer from spinal TB
infection. Half have lesions in the spine with
neurologic deficits 10 % - 45% of sufferers
Matrix metalloproteinase (MMP) is a zinc-
dependent protease, which plays a role in the process
of degradation of the extracellular matrix and
modulates the inflammatory response by facilitating
and inhibiting different cytokines. Research shows
that MMP-1 is the main collagenase in TB patients,
and the expression of MMP-1 is suppressed by p-
aminosalicylic acid, which is an anti-tuberculous
agent that has been used for 70 years.
Two gelatinases, MMP-2 and MMP-1, have the
ability to reduce original IV collagen and
denaturation of type I collagen (gelatin). Both
circulation and resident inflammatory cells have the
capacity to synthesize MMP-1. Research into
experimental studies has provided evidence that the
MMP-1 level was significantly higher in the
bronchoalveolar fluid of patients with active cavitary
tuberculosis, and lung extract of mice infected with
M. tuberculosis, compared to the control group. An
increased significance of MMP-1 was also observed
in cerebrospinal fluid (CSF) in tuberculous
meningitis patients and also compared with people
suffering from viral meningitis, where usually these
enzymes are not usually found in cerebrospinal fluid.
There are previous studies shown that M.
tuberculosis can stimulate the expression of MMP-1
Siregar, A., Siregar, O. and Moesbar, N.
Comparison of Serum MMP-1 Value Levels in Spondylitis Tuberculose with Degenerative Spine Disease.
DOI: 10.5220/0009840900050008
In Proceedings of the 2nd International Conference on Tropical Medicine and Infectious Disease (ICTROMI 2019), pages 5-8
ISBN: 978-989-758-469-5
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5
in the lungs of infected organisms, but no studies have
examined how the expression of MMP-1 in
tuberculous spondylitis when compared with the
control group, therefore researchers are interested in
trying evaluating and comparing the expression of
MMP-1 in tuberculous spondylitis using serum levels
in the blood of patients suffering from tuberculous
spondylitis and compared to the control group, in this
case the control group in this study were patients with
degenerative diseases of the spine.
2 PRELIMINARY
Before discussing the results of the study, because this
study had never been done before, it was conducted
with a small scale preliminary study using 8 balanced
subjects with 4 subjects (4 subjects with tuberculous
spondylitis, 4 subjects with the degenerative spine) to
obtain a mean and standard deviation from each one
group.
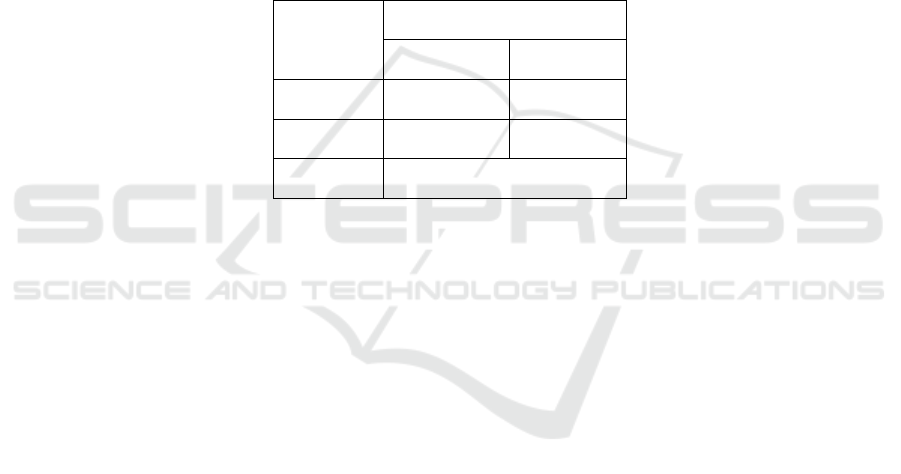
Table 1. Shows that the distribution of tuberculous
spondylitis samples and spinal degenerative diseases
in the preliminary study was as many as 8 subjects
with 5 women (62.5%) and men as many as 3
(37.5%). With a mean and standard deviation of 38.63
± 18.24 years. It shows that the mean of subjects with
spinal degenerative disease 750 ± 288.67 and mean
tuberculous spondylitis 1125 ± 629.15.
Table 1: ST, Spondylitis Tuberculosis; DSD, Degenerative Spine Disease;
Variable
Type of Disease
ST
DSD
Gender M/F
2/2
1/3
MMP-1
1125 ± 629.15
750 ± 288.67
Mean Age
38.63 ± 18.24
3 METHOD
The sample size is calculated based on the categorical
numerical unpaired analytic formula, the minimum
sample size can be obtained as many as 17.04 ~ 18
subjects. All data are processed and presented in a
table form and then further classified into 1) general
description, 2) demographic description of the
subjects of the serum MMP-1 value in tuberculous
spondylitis with degenerative disease in the spine 3)
normality test data on serum MMP-1 values
tuberculous spondylitis with degenerative diseases of
the spine, 4) analysis of the influence of whether there
are differences in serum MMP-1 values in
tuberculous spondylitis with degenerative diseases of
the spine.
4 RESULTS
From the results of the statistical analysis of the
comparison of serum MMP-1 values in tuberculous
spondylitis (ST) with degenerative spine disease
(DSD), the results were significant that the serum
MMP-1 value was greater and this was indicated by a
p-value of 0.016 ( p <0.05). Table 3. Distribution of
the results of serum MMP-1 levels in tuberculous
spondylitis and spinal degenerative diseases 18
subjects, diagnosed with 9 tuberculosis spondylitis
(50%) and 9 degenerative spinal diseases (50%) with
mean and standard the deviation of the serum MMP-
1 tuberculosis spondylitis level was 1055.56 ± 390.86
while the mean and standard deviation of serum
MMP-1 values in spinal degenerative diseases was
666.67 ± 250.00. From the results of the statistical
analysis of the comparison of serum MMP-1 values
in tuberculous spondylitis (ST) with degenerative
spine disease (DSD), the results were significant that
the serum MMP-1 value was greater and this was
indicated by a p-value of 0.016 ( p <0.05).
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
6
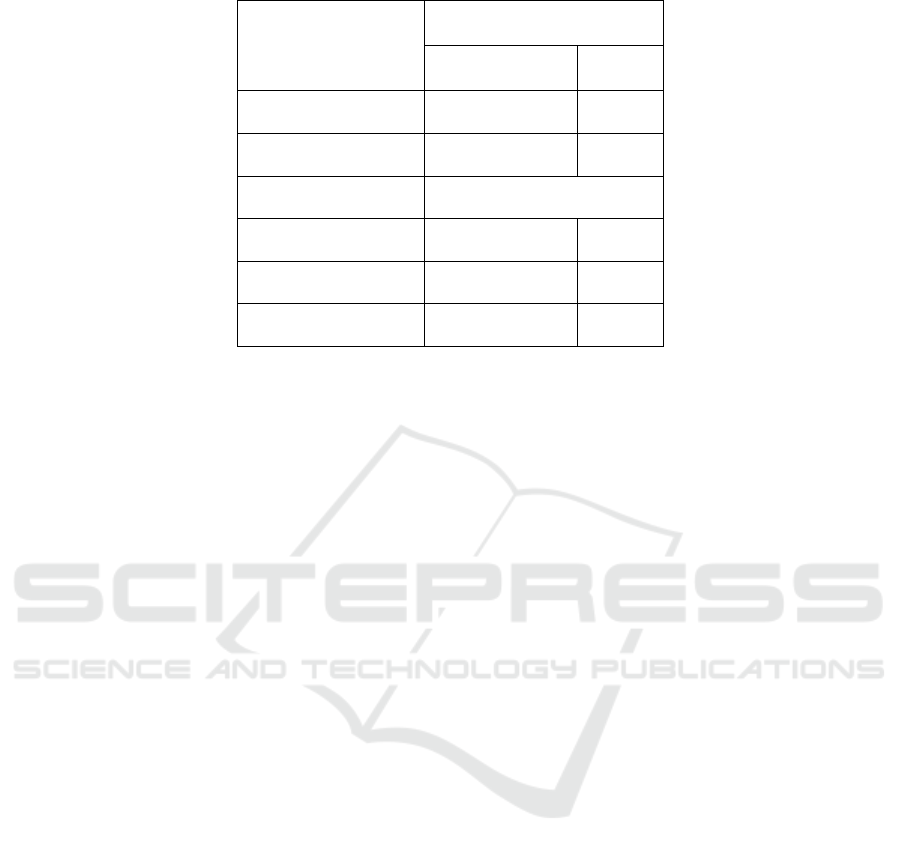
Table 2: ST, Spondylitis Tuberculosis; DSD, Degenerative Spine Disease.
Variable
Type of Disease
ST
DSD
Mean MMP-1 (nm/ml)
1055.56
666.67
SD
± 390.86
± 250.00
p-Value
0.016
Older Age
13 years
43 years
Younger Age
62 years
73 years
TB Drug Consumption
8.89 ± 4.70 weeks
0
5 DISCUSSION
The main objective of this study was to determine
whether there was a difference between serum MMP-
1 levels in spondylitis tuberculosis and degenerative
diseases of the spine. In a previous study conducted
by Hrabec et al., 2002 also found that serum MMP-1
levels in patients with active pulmonary tuberculosis
had significantly higher levels of the control group.
This finding is in accordance with the study
conducted that there were significant differences in
MMP-1 levels in patients with spondylitis
tuberculosis with the control group.
6 CONCLUSION
MMP-1 values in spondylitis tuberculosis were
significant and greater compared to degenerative
spine disease and this was indicated by a p-value of
0.016 (p <0.05).
CONFLICT OF INTEREST
Non declared in this study.
REFERENCES
American Thoracic Society. Diagnostic standards and
classification of tuberculosis in adults and children. Am
J respire Crit. 2000 ; 161 : 1376-1395.
Spiegel DA, Singh GK, Banskota AK. Tuberkulosa of the
Musculoskeletal System. Techniques in Orthopaedics.
2005 ; 20 (2) : 167-178.
Harisinghani M G, McLoud T C, Shepard J, et al.
Tuberkulosa from Head to Toe. Radiographics. 2000;
20: 449-470.
Agarwal P, Rathi P, Verma R, Pradhan CG. Tuberculous
spondylitis: "Global lesion”. Special issues on
Tuberkulosa. Bombay Hospital Journal. 1999.
Leibert E, Haralambou G. Tuberkulosa. In: Rom WN and
Garay S, eds. Spinal tuberculosis. Lippincott, Williams,
and Wilkins. 2004: 565–577.
Zhang Q, Hui W, Litherland GJ, Barter MJ, Davidson R,
Darrah C, et al. Differential Toll-like receptor-
dependent collagenase expression in chondrocytes.
Ann Rheum Dis 2008;67: 1633–41.
López-Otín C, Palavalli LH, Samuels Y. Protective roles of
matrix metalloproteinases: from mousemodels to
human cancer. Cell Cycle 2009;8: 3657–620.
Venkateshwari A, Sri Manjari K, Krishnaveni D,
Nallari P, Vidyasagar A, Jyothy A. Role of plasma
MMP 9 levels in the pathogenesis of chronic
pancreatitis. Indian J Clin Biochem 2011;26: 136–9.
Savant C, Rajamani K. Tropical Diseases of the Spinal
Cord. In: Critchley E, Eisen A., editor. Spinal Cord
Disease: Basic Science, Diagnosis and Management.
London: Springer-Verlag, 1997: 378-87.
Tachdjian, M.O. Tuberkulosa of the spine. In: Pediatric
Orthopedics.2nd ed. Philadelphia: W.B. Saunders,
1990: 1449-54.
Lindsay, KW, Bone I, Callander R. Spinal Cord and Root
Compression. In: Neurology and Neurosurgery
Illustrated. 2nded. Edinburgh: Churchill Livingstone,
1991: 388.
Graham JM, Kozak J. Spinal Tuberkulosa. In: Hochschuler
SH, Cotler HB, Guyer RD., editor. Rehabilitation Of
The Spine: Science and Practice. St. Louis: Mosby-
Year Book, Inc., 1993: 387-90.
Comparison of Serum MMP-1 Value Levels in Spondylitis Tuberculose with Degenerative Spine Disease
7

Lauerman WC, Regan M. Spine. In: Miller, editor. Review
of Orthopaedics. 2nd ed. Philadelphia: W.B. Saunders,
1996: 270-91.
Currier B.L, Eismont F.J. Infections of The Spine. In: The
Spine. 3rd ed. Rothman Simeone editor. Philadelphia:
W.B. Sauders, 1992: 1353-64.
Ombregt L, Bisschop P, Ter Veer H.J, Van de Velde T.
Non-Mechanical Disorders of The Lumbar Spine. In: A
System of Orthopaedic Medicine.Philadelphia: W.B.
Saunders, 1995: 615-32.
Natarajan M, Maxilvahanan. Tuberkulosa of the spine. In:
http:/www.bonetumourorg.
/book/APTEXT/intex.html. Book of orthopedics and
traumatology.
Miller F, Horne N, Crofton SJ. Tuberkulosa in Bone and
Joint. In: Clinical Tuberkulosa.2nd ed.: London:
Macmillan Education Ltd, 1999: 62-6.
Wood.G.W. Infections of Spine. In: Campbell’s Operative
Orthopaedics. 7th ed. Crenshaw A.H editor. St. Louis:
C.V. Mosby Company, 1987: 3323-45.
ICTROMI 2019 - The 2nd International Conference on Tropical Medicine and Infectious Disease
8
