
The Effect of Ginger (Zingiber Officinale Roscoe) Fractionation in
Decreasing Uric Acid Level of Hyperuricemic White Mice
Tika Afriani
1
, Rizky Yulion
1
, Marlina Andriani
1
, Fahma Syufyani
2
, Darma Fadri
1
1
Department of Pharmacy, Mohammad Natsir University, Bukittinggi 26136, Indonesia
2
Medistra Health Institut, Lubuk Pakam, North Sumatera, Indonesia
Keywords: uric acid, ginger (Zingiber officinale Roscoe), fractionation, chicken liver, allopurinol
Abstract: This study aims to determine the effects of ginger fractionation on uric acid level of
hyperuricemic white mice. The study was conducted in 7 days using an in vivo experimental
method. Ginger was extracted with 70% ethanol and subsequently fractionated by using n-
hexane, ethyl acetate, and water residual solvents. The mice divided into six group treatments.
Doses used of the fraction n-hexane, ethyl acetate, and water residual were 100 mg/kg of body
weight and allopurinol as a standard was 10 mg/kg of body weight. The examination was carried
out on mice with hyperuricemic induced by fresh chicken liver juice 0.5 ml/20g of body weight
administered orally. The uric acid levels were measured by NESCO
®
Multi Check Digital tools.
The result of this study showed that ethyl acetate fraction doses 100 mg/kg of body weight are
the best fraction to lowering level of uric acid in blood serum.
1 INTRODUCTION
Gout is one of noncontagious disease that has high
prevalence in the world. Gout is considered to be
primarily a male disease, but there is a more equal
sex distribution among elderly patients (Pokhrel et
al., 2011). Based on World Health Organization
(WHO) survey, Indonesia is the largest state number
4 in the world whose population is suffering from
gout and uric acid disease is estimated to occur in
840 people of every 100,000 people. The prevalence
of uric acid disease in Indonesia occurs at age below
34 years old by 32% and above 34 years old by 68%
(WHO, 2015). In Indonesia, hyperuricemic attacking
people under 34 years old with prevalence 1.6-
13.6/100.000, and it is predicted keep increasing day
by day (Thayibah et al., 2018).
Gout is caused by increased levels of uric acid in
the blood (hyperuricemia) exceeding normal levels,
giving rise to needle-shaped uric acid crystals and
causing stiffness and inflammation in the joints.
Hyperuricemia is a condition where the kidneys fail
to excrete uric acid, resulting in high uric acid levels.
Hyperuricemia is a considered to be closely
associated with increased risk for developing gout,
cardiovascular diseases, hypertension, and metabolic
syndrome (Chen Yu-Chen et al., 2014)
High levels of uric acid are caused by the
deposition of monosodium crystals due to the
breakdown of purines or combination of both.
Normal uric acid levels in the blood range between
3.6 - 8.2 mg / dl in men and 2.3 - 6.1 mg / dl in
women. High level of uric acid serum caused by
disturbances metabolism of purine in the body,
heredity, lifestyle, and consume food containing
high purine, for example meat, shells and viscera
(Misnadiarly, 2017).
Everyone has uric acid in his body which in
normal metabolic condition used to produce uric
acid and the amount of uric acid must not exceed
normal. Uric acid provided by our body about 85
percent and it is mean that the body only needs
about 15 percent purine from food. If the body
consume high contain purine from food, it causing
gout disease and if can progress in to worse
condition into coronary heart disease because crystal
of uric acid in endhotelium can blocked blood
vessels. Therefore high uric acid levels should be
treated so that the impact does not fall in to worse
condition (Indriawan, 2010).
Afriani, T., Yulion, R., Andriani, M., Syufyani, F. and Fadri, D.
The Effect of Ginger (Zingiber Officinale Roscoe) Fractionation in Decreasing Uric Acid Level of Hyperuricemic White Mice.
DOI: 10.5220/0009838404670474
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 467-474
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
467

A variety of treatment techniques have been
used to treat gout, such as the use of Non-Steroidal
Anti-Inflammatory compounds (NSAIDs) and the
use of Xanthine Oxidase (XOD) inhibiting
compounds to reduce uric acid production.
Allopurinol is currently the most effective XOD
inhibitor, which is used for treating hyperuricemic
and gout by reducing circulating levels of uric acid
and vascular oxidative stress. However, serious side
effects include skin rashes and allergic reactions that
occur in some clinical patients (Chen Yu-Chen et al.,
2014). The emergence of side effects and various
deficiencies in modern medicine demands to find
drugs with the smallest possible side effects, one of
alternative to this situation is to use traditional
medicine.
Ginger is one of the herbs used for traditional
medicine in treating gout. Ginger (Zingiber
officinale Rosc) is a potential herbal plant according
to RISTOJA data in 2015 that is used by Indonesian
people for lowering of uric acid levels in the blood.
There are 178 plants used in the herb for
rheumatism/gout and ginger (Zingiber officinale
Rosc) is the most widely used herbal plant as a herb
to lower uric acid complaints in Indonesia. In
addition, ginger extract has also been done a
scientific search, get the result that ginger ethanol
extract (Zingiber officinale Rosc) can be potential as
anti hyperuricemia (Lallo, et al., 2018; Yulion, et al.,
2017) where the data supports this research.
Jolad et al. (2014) identified more than 60
compounds in fresh ginger grouped into two broader
categories; volatiles and non-volatiles. Volatile
compounds including sesquiterpene and mono
terpenoids hydrocarbons providing the distinct
aroma and taste of ginger and non-volatile
compounds include gingerols, shogaols, paradols
and zingerone.
Ginger contain secondary metabolite such as
phenolic compound, flavonoid which is can
lowering level of uric acid in the blood serum
(Andriani, 2018). The mechanism for lowering uric
acid levels is by inhibiting the conversion of
xanthine into uric acid. The level of uric acid can be
derived from excessive production or less excretion
through the kidney (Facchini, et al., 1991). This
study aims to determine the effect of ginger
fractionation (Zingiber officinale Rosc) for 7 days on
serum uric acid levels of mice induced by chicken
liver juice.
The absence of therapy that was found safe
and quick to patients with gout while patients with
gout is in reproductive age in productivity and
patients with gout is very big amount in Indonesia
that based on the above information then researchers
interested in doing research ginger (Z. officinale
Rosc) in lowering uric acid and in the form of
fractionate. From this research will be proven that
fractionation of ginger (Z. officinale Rosc) able to
lower the levels of uric acid in 7 day (
Lembaga Ilmu
Pengetahuan Indonesia, 2016).
The research was conducted to obtain activity
extract ethanol on antihiperurisemia in fractionate
with ethyl acetate, n-hexsan and fraction of water
from ginger (Zingiber officinale Rosc) using animal
testing male mice (Mus musculus) to be raised levels
uric acid first with induced chicken liver juice and to
compare positive impact with using allopurinol.
2 RESEARCH METHODOLOGY
Materials and Methods
The materials used in this study were chicken
liver juice, ethanol 70% (bratachem), ethyl acetate
(bratachem), n-hexsane (bratachem), aqua destilata
(bratachem), food standard Squeaky, allopurinol
(Kimia Farma), Na-CMC, Nesco
®
, and test strips
uric acid Nesco
®
.
Plant materials
Gingers (Z. officinale Rosc) were collected
from Sariak Laweh Sub-district of West Sumatra
province Agam Regency. This species was identified
by ANDA Herbarium Department of Biology
Faculty of Mathematics and Natural Sciences
University of Andalas.
Preparation of extracts
Ginger (Z. officinale Rosc) were washed well
to remove the dust and other foreign materials, dried
and grounded into tea size powder. The powder was
extracted by maceration with ethanol 70% for
overnight at room temperature. This process was
repeated two times with the same type and amount
of solvent. The extracts were combined and
evaporated by using rotary evaporator (Buchi
Rotavapor R-200) under reduce pressure to obtain
ethanol crude extract (Departemen Kesehatan,
2008).
Fractionation procedures
The ethanol crude extract from Z. officinale
Rosc was dissolved in distillated water. Then it was
extracted successively with different organic
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
468

solvents which were hexane and ethyl acetate with
ratio 1:1 (v/v) in the separatory funnel. The funnel
was shaken for 2 minutes and left at room
temperature for 2 hours to settle down. The
procedure was repeated for several times to obtain
hexane, ethyl acetate, and residual water fractions.
All crude extracts were evaporated completely by
using rotary evaporator (Buchi Rotavapor R-200)
under reduce pressure to obtain dry crude extracts
(Arifin, Fahrefi, & Dharma, 2013; Ningdyah,
Alimuddin, & Jayuska, 2015; Parwata, Rita, &
Yoga, 2009; Tahir, Saleh, & Pasaribu, 2013).
Treatment of experimental animals and dosing
design
The mice were divided into six groups
containing seven animals in each with different
treatments on each group and given variation of
fraction with doses 100mg/kgBB in 7 days. A total
of 42 male mice with age 3-4 m and weighing 20-30
g were used for this study. The mice adapted for 7
days with the environment at constant temperature
before used as experimental objects. They were
given feed standard pellets and water during the
study. The mice were considered being healthy if the
difference of their weight before and after adaptation
less than 10% and indicates normal behavior
visually (Vogel, 2008).
Male mice were selected for this study based
on consideration that male mice are lack of estrogen.
This condition makes stress level of male mice
lesser than female mice, a factor that might
disruptive during the study period. And if they’ve
estrogen, the amount of this hormones exist only in
relatively few (Suhendi, Nurcahyanti, Muhtadi, &
Sutrisna, 2011).
Making inducers from chicken liver
Chicken liver juice used as inducers to make
hyperuricemic condition. The inducers given to mice
with oral using sonde with doses 25ml/kgBB
(Hayani dan Widyaningsih, 2011). Chicken liver is
one of food that have high contain of purine making
it suitable for hyperuricemic condition. Chicken
liver contain 150-1000 mg purine per 100g liver and
able to induce high uric acid levels.
Standard
Standard in this study using allopurinol, which is an
antigout drug. Allopurinol used as standard with
doses 10 mg/kgBB.
Antihyperuricemic activity
Preliminary study purpose to know the fraction that
has the best activity in lowering uric acid levels in
experimental animal blood. The mice were divided
into six groups containing seven animals in each
with different treatments on each group, as seen in
the following table:
Table 1. Group Treatments
No Group Treatment
1
Negative Control Administered orally solution
of Na-CMC 0.5%
2
Positive Control Induced by chicken liver
juice 0.5 ml/20g bw
3
Hyperuricemia +
ginger n-hexane
fraction
Induced by chicken liver
juice 0.5 ml/20g bw and n-
hexane fraction dose 100
mg/kg bw
4
Hyperuricemia +
ginger ethyl acetate
fraction
Induced by chicken liver
juice 0.5 ml/20g bw and
ethyl acetate fraction dose
100 mg/kg bw
5
Hyperuricemia +
remaining fraction of
ginger water
Induced by chicken liver
juice 0.5 ml/20g bw and
water residual fraction dose
100 mg/kg bw
6
Hyperuricemia +
allopurinol
Induced by chicken liver
juice 0.5 ml/20g bw and
allopurinol dose 10 mg/kg
bw
At the early stage, all experimental animals are
conditioned to hyperuricemia except negative
control, using inducer chicken liver juice doses 0.5
mL/20g of body weight with oral administration of
Probe until the level of blood acidity increase. The
process of hyperuricemic condition carried out for
seven days. Fraction was given 1 hour after
induction process of hyperuricemic (Suhendi,
Nurcahyanti, Muhtadi, & Sutrisna, 2011) and then
uric acid levels measured everyday using NESCO
®
Multi Check Digital tools it done 2 hour after
fraction was given (Muhtadi, Suhendi, W., &
Sutrisna, 2013).
Statistical analysis
The statistically significant of difference was
calculated by the analysis of variance followed by
Duncan’s Multiple Range Test.
3 RESULTS AND DISCUSSION
A total of 600 grams of rhizomes have been dried,
refined and then extracted with ethanol 70%
producing a condensed extract of 160.5 grams. The
basic principle is to grind the plant material (dry or
wet) finer, which increases the surface area for
extraction thereby increasing the rate of extraction
The Effect of Ginger (Zingiber Officinale Roscoe) Fractionation in Decreasing Uric Acid Level of Hyperuricemic White Mice
469

(Pandey and Tripathi, 2014). The pieces should not
be too big, otherwise the solvent will not be able to
penetrate the innermost cells. They also should not
be reduced to powder, that would result in losing the
volatile active ingredients contained inside the plant,
and also losing the difficult separation by filtration
of the plant material from the liquid used once
maceration is completed.
The solvent must be chosen based upon the
chemical nature of the compounds contained within
the plant. Since the end product will contain traces
of residual solvent, the solvent should be nontoxic
and should not interfere with the bioassay (Pandey
and Tripathi, 2014). Ethanol has been known as a
good solvent for polyphenol extraction and is safe
for human consumption (Quy Diem Do et al., 2014).
The reasons for the selection solvent consisting of
ethanol by 70% and water by 30% because ethanol
70% volatile, cheap, can afford and safe (Azis, et al.,
2014). The obtained yield was 26.75%. Results of
the result obtained, above the value stated in the
Indonesian Herbal Pharmacopeia is not less than
6.6% (Departemen Kesehatan, 2008).
Sample 600 grams dry extracted, with the
purpose to separate compounds based on their
relative solubilities in two different immiscible
liquids, usually water and an organic solvent
(Endarini, 2016). Extraction is the separation of
medicinally active portions of plant (and animal)
tissues from the inactive/inert components using
selective solvents through standard procedures.
During extraction, solvents diffuse into the solid
plant material and solubilize compounds with
similar polarity (Sruthi and Indira, 2016).
Technique extraction that used in research it is
technique maceration because this technique
relatively simple does not require an apparatus
complicated and can avoid all compound due to heat
components (Efendi, 2018). Maceration is an
extractive technique that is conducted at room
temperature. It consists of immersing a plant in a
liquid (water, oil, alcohol, etc.) inside an airtight
container, for a variable time based on the plant
material and liquid used. In maceration (for fluid
extract), whole or coarsely powdered plant-drug is
kept in contact with the solvent in a stoppered
container for a defined period with frequent agitation
until soluble matter is dissolved. At the end of the
process the solvent is drained off and the remaining
miscella is removed from the plant material through
pressing or centrifuging (Pandey and Tripathi, 2014;
Silva et al, 2017).
A total of 50 grams of condensed extracts of
ginger leaves are fractionated consecutively by using
polar, semi-polar, and nonpolar solvents. The
ethanol extract was fractionated with water and n-
hexane to obtain semi-polar fraction. The water
layer was fractionated with ethyl acetate solvent to
obtain semi-polar fraction of ethyl acetate and polar
water fraction. In principle, the polar solvents
dissolve polar solutes and nonpolar solvents dissolve
non polar solutes that are also called “like dissolve
like” (Harborne, 1987; Mariana, Andayani, &
Gunawan, 2013). The amount of viscous fraction
obtained is n-hexane 4.22 gram, ethyl acetate 5.51
gram, and water remaining 27.5 gram with a yield
percentage of 8.44%; 11.01% and 55% respectively.
Hyperuricemia is an elevated uric acid level
in the blood. This elevated level is the result of
increased production, decreased excretion of uric
acid, or a combination of both processes.
Hyperuricemia can lead to gout and nephrolithiasis
(George and Minter, 2019). Uric acid is formed
when purines break down in your body. Purines are
chemicals found in certain foods include all meats
but spesifically organ meats, game meats and some
seafood.
For hyperuricemic condition, the mice were
given chicken liver juice as inducers because
chicken liver contain high level of purine (≥300
mg/100 g). Chicken liver juice used as inducer of
uric acid with the dose is 0.5 mL/20g of body weight
for animal experiments until the level of uric acid
increase (Juwita et al., 2014).
Allopurinol used as standard in this study
because allopurinol is widely known as a synthetic
drug widely used for hyperuricemic. Allopurinol is
an alternative compound which used to increase uric
acid excretion through inhibition of the xanthine
oxidase enzyme and 80% is absorbed after oral
administration. Like uric acid, allopurinol itself is
metabolized by xanthine oxidase to allantoxanthine,
maintains the capacity to prevent xanthine oxidase
and has along enough effect duration so that the
treatment is sufficient once a day (Katzung, 2007).
This study using 42 male white mice with
average weight 20-30 grams and average age 3-4
months. The acclimatization procedurs was carried
out in 7 days so that the animals can adaptation with
the environment. The mice must be healthy, showing
normal behaviour and have shining clear eyes.
During maintenance mice were feed and drinking
enough (Malole, 1989).
After acclimation, treatments animals divided
into six groups with each groups consisting of seven
mice. Three groups animals given variation of types
fraction such as n-hexane, ethyl acetate, and residual
water with doses 100mg/kgBB in seven days as
testing groups. One group animals as negative
control which were not received any treatment, one
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
470
group as positive control which were induced by
chicken liver juice 0.5 ml/20g bw, and one group
given chemical drugs allopurinol as standard.
Fraction was given 1 hour after induction process of
hyperuricemic, and then uric acid level measured
everyday using NESCO
®
Multi Check Digital tools
it done 2 hour after fraction was given.
Table 2. Effects of Zingiber officinale Rosc fractions on
level of uric acid serum in mice induced chicken liver
juice
Grou
p/day
1 2 3 4 5 6 7
Nega
tive
6,8
±
2,3
6,01
±
2,0
6,3
±
1,9
4,4
±
1,7
5,8
±
1,4
5,0
±
1,5
5,3
±
1,9
Positi
ve
9,4
±
2,9
9,3 ±
2,8
9,2
±
2,6
7,4
±
3,9
6,5
±
3,3
7,4
±
2,8
7,1
±
2,0
n-
Heks
an
6,9
±
2,2
6,5 ±
1,6
5,6
±
1,7
4,4
±
1,3
4,1
±
1,0
4,2
±
1,0
3,9
±
0,6
Ethyl
Aseta
te
5,7
±
2,1
5,3 ±
1,2
4,8
±
1,0
4,1
±
0,8
3,7
±
0,5
3,8
±
0.6
3,9
±
0,4
Wate
r
6,0
±
2,4
5 ±
1,5
5,6
±
0,9
4,7
±
1,7
4,2
±
1,7
4,1
±
1,2
4,4
±
0,9
Allop
urino
l
6,7
±
2,4
5,5 ±
1,6
5,7
±
1,3
9,1
±
4,5
5,1
±
2,2
5,8
±
2,1
5 ±
1,3
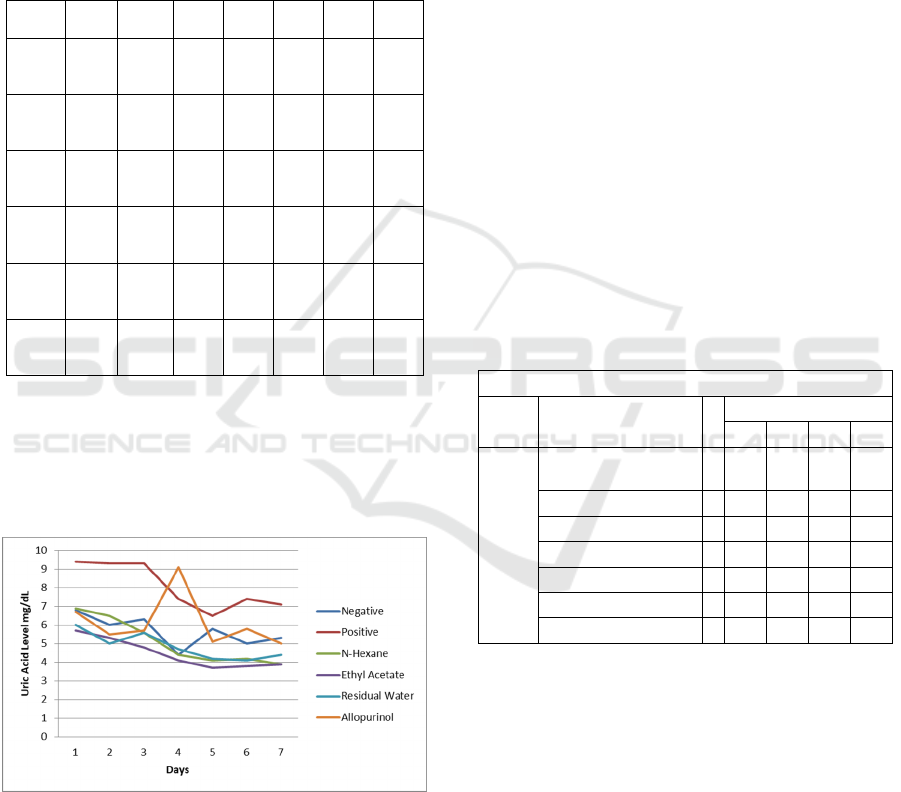
From the above table it can be concluded that
the ethyl acetate fraction is the best fraction that can
reduce uric acid levels in mice compared to the n-
hexane fraction and residual water.
Figure 1. The effect of ginger (Zingiber officinale Rosc)
fractionation in decreasing uric acid level
The effect of ginger (Zingiber officinale
Rosc) fractionation in decreasing uric acid level of
hyperuricemic white mice show at figure 1. In the
Figure 1, we can see the level of uric acid decrease
in every fraction.
Ginger (Zingiber officinale Rosc) contains
over 400 different compounds. The mayor
constituents are carbohydrates (50-70%), lipids (3-
8%), terpens, and phenolic compounds (Prasad and
Tyagi, 2015). Flavonoid is a group of plant phenolic
compound that act as antioxidant such as quercetion,
rutin, catechin, epicatechin, kaempferol and
naringenin (Ghasemzadeh et al, 2010). Due to their
importance in human health, ginger can be used to
pharmacological activites like anti inflammation,
anticancer, antioxidant, antiplatelet, and can be used
to reduce cholesterol and uric acid level (Rehman,
2011).
Flavonoid functions as an inhibitor in uric
acid formation and able to reduce the level of uric
acid by blocking xanthine oxidase, the enzyme
responsible for regulating uric acid formation.
In this study, variation of fraction used to
knowing which the best fraction to lowering level of
uric acid. The fractionation process was carried out
in stages based on level of polarity, from polar, semi
polar and nonpolar solvents. From the experiment,
Duncan’s Multiple Range Test used to determine the
best fraction for lowering uric acid level.
Table 3. Duncan’s Multiple Range Test of Uric Acid
Level
Uric Acid Activity
Group N
Subset
1 2 3 4
Duncan
a
Ethyl Acetate
100mg/kgBB
49 4.451
Water 100mg/kgBB 49 4.865 4.865
n-Hexane 100mg/kgBB 49 5.067 5.067
Negative Control 49 5.667 5.667
Allupurinol 10mg/kgBB 49 6.135
Positive Control 49 8.031
Sig. .150 .060 .247 1.000
Test results from analysis Duncan’s
Multiple Range Test showed that the best fraction
for lowering level of uric acid serum is ethyl acetate
fraction, because the statistical value of ethyl acetate
has different subset with other test groups. It means,
suspected active compounds that are given can lower
the best of uric acid levels in the blood is in the
semi-polar fraction. While on the allupurinol showed
that the effect is not different significantly with ethyl
acetate. It because the dose of the fraction that used
in this study is greater than the dose of Allupurinol.
The Effect of Ginger (Zingiber Officinale Roscoe) Fractionation in Decreasing Uric Acid Level of Hyperuricemic White Mice
471

4 CONCLUSIONS
A total of 600 grams of rhizomes have been dried,
refined and then extracted with ethanol 70%
producing a condensed extract of 160.5 grams. A
total of 50 grams of condensed extracts of ginger
leaves are fractionated consecutively by using n-
hexane, ethyl acetate, and residual water. The
amount of viscous fraction obtained is n-hexane 4.22
gram, ethyl acetate 5.51 gram, and water remaining
27.5 gram with a yield percentage of 8.44%; 11.01%
and 55% respectively. From the study can conclude
that ethyl acetate fraction of Zingiber officinale Rosc
is the best fraction for lowering level of uric acid
serum. For the future study we can use variation of
doses, pathology with other diseases or isolation of
pure compound from ginger.
ACKNOWLEDGEMENTS
We thanks to RISTEKDIKTI was funded this series
of research through a beginner lecturer research
program. We hope this series of research can be
continued to the next stage.
REFERENCES
Andriani, A. (2018). Pengaruh Pemberian Air Rebusan
Daun Salam (Syzygium Polyanthum) Terhadap
Penurunan Kadar Asam Urat. Jurnal Ipteks Terapan,
12(3), 222. https://doi.org/10.22216/jit.2018.v12i3.430
Arifin, H., Fahrefi, M., & Dharma, S. (2013). Pengaruh
Fraksi Air Herba Seledri (Apium graveolens L.)
Terhadap Kadar Kolesterol Total Mencit Putih Jantan
Hiperkolesterol. In Prosiding Seminar Nasional
Perkembangan Terkini Sains Farmasi dan Klinik III
(pp. 293–304). Padang.
Azis, T., Febrizky, S., & Mario, D. A. (2014) “Pengaruh
Jenis Pelarut tehadap Persen Yield Alkaloid dari
Daun Salam India”. Universitas Sriwijaya.
Chen, Yu-Chen, Huang, Chang-Chi, Tsai, Chang-Keng,
Huang, Jan-Wei, Huang, Ching-Wen, Hsu, Chen-Yu,
and Hsu, Lin-Feng. (2014). Evaluation of the
Antihyperuricemic Activity of Phytochemicals from
Davallia formosana by Enzyme Assay and
Hyperuricemic Mice Model. Evidence-Based
Complementary and Alternative Medicine.
Departemen Kesehatan Republik Indonesia. Parameter
Standar Umum Ekstrak Tumbuhan Obat. Jakarta:
Direktorat Jendral Pengawasan Obat dan Makanan;
2000:10-11.
Departemen Kesehatan. (2008). Farmakope Herbal
Indonesia (Edisi 1). Jakarta: Departemen Kesehatan.
Departemen Kesehatan. (2017). Farmakope Herbal
Indonesia (Edisi 2). Jakarta: Departemen Kesehatan.
Endarini, L. H. (2016). Farmakognosi dan Fitokimia.
Jakarta : Pusdik SDM Kesehatan Badan
Pengembangan dan Pemberdayaan Sumber Daya
Manusia Kesehatan.
Facchini, F., Chen, Y. D., Hollenbeck, C. B., & Reaven,
G. M. (1991). Relationship between resistance to
insulin-mediated glucose uptake, urinary uric acid
clearance, and plasma uric acid concentration. Jama,
266(21), 3008–3011.
https://doi.org/10.1001/jama.266.21.3008
Efendi Juniar Ikhsan. (2018). Uji Aktivitas
Antihiperurisemia ekstrak daun kemangi (ocimum
sanctum) dan daun salam (Syzygium polyanthum)pada
tikus yang diinduksi hati ayam. Naskah publikasi.
George, Christina and Minter, A., D. (2019).
Hyperuricemia. MSU-Mclaren Oakland.
Ghasemzadeh A, Jaafar HZ, Rahmat A. 2010. Antioxidant
activities, total phenolic and flavonoids content in two
varieties of Malaysia young ginger (Zingiber officinale
Roscoe). Molecules. 15(6): 4324-4333.
Hamzah, L., Arifin, H., & Ahmad, A. (2014). Pengaruh
Ekstrak Etanol Rambut Jagung (Zea Mays, L)
Terhadap Kadar Asam Uratdarah Mencit Putih Jantan
Hiperurisemia. In Perkembangan Terkini Sains
Farmasi dan Klinik IV (pp. 282–293). Padang.
Harborne, J. (1987). Metode Fitokimia, Penentun Cara
Modern Menganalisis Tumbuhan. (S. I. Padmawinata
K, Trans.) (2nd ed.). Bandung: ITB.
Hassan F. Al-Azzawie and Samah A.Abd. 2015. Effects
of Crude Flavonoids from Ginger (
Zingiber
officinale), on Serum Uric Acid Levels, Biomarkers of
Oxidative Stress and Xanthine Oxidase Activity in
Oxonate-Induced Hyperuricemic Rats. Dept. of
Biotechnology/College of Science/Baghdad
University/Iraq
Hayani, M., Widyaningsih, W. (2011). Efek Ekstrak
Etanol Herba Putri Malu (Mimosa pudica) sebagai
Penurun Kadar Asam Urat Serum Mencit Jantan Galur
Swiss. Prosiding Seminar Nasional "Home Care".
Hawking, D. W., & Rahn, D. W. (2005).
Pharmacotherapy: A Pathophysiologic Approach. (J.
T. DiPiro, R. L. Talbert, G. C. Yee, G. R. Matzke, B.
G.Wells, & L. M. Posey, Eds.) (6th Editio). United
States of America: The McGraw-Hill Companies, Inc.
Hernani dan E. Hayani. Identification of chemical
components on red ginger (Zingiber officinale var.
Rubrum) by GC-MS. Proc. International Seminar on
natural products chemistry and utilization of natural
resources. Jakarta: UI-Unesco; 2001 :501-505
Indriawan.(2010).Penyakit.asamurat/gout.unikom.ac.id/re
po/sector/kampus/view/blog/key/.../Penyakit.
Jolad, S. D., Lantz, R. C., Solyom, A. M., Chen, G. J.,
Bates, R. B. and Timmerman,B. N. (2004) Fresh
organically grown ginger (Zingiber officinale)
(composition and effects on LPS-induced PGE2
production, Phytochem., vol. 65 : 1937–1954.
Juwita, D. A., Arifin, H., & Popy Handayani. (2014).
Pengaruh Fraksi Air Herba Seledri (Apium graveolens
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
472

L.) Terhadap Kadar Asam Urat Mencit Putih Jantan
Hiperurisemia. In Seminar Nasional dan Workshop
“Perkembangan Terkini Sains Farmasi dan Klinik IV”
(pp. 187–191).
Katzung BG, Masters SB, Trevor AJ. (2007). Basic and
clinical pharmacology. 10
TH
ed. New York: McGraw
Hill Press.
Kristiani, R.D., Rahayu, D dan Subarnas, A. 2013.
Aktivitas Antihiperurisemia Ekstrak Etanol Akar Pakis
Tamgkur (Polypodium feei) Pada Mencit Jantan.
Bionatura-Jurnal Ilmuilmu Hayati dan Fisik. Vol 15
(3):156-159.
Lallo, S., Mirwan, M., Palino, A., Nursamsiar, &
Hardianti, B. (2018). Aktifitas Ekstrak Jahe Merah
Dalam Menurunkan Asam Urat Pada Kelinci Serta
Isolasi Dan Identifikasi Senyawa Bioaktifnya. Jurnal
Fitofarmaka Indonesia, 5(1), 271–278.
Lembaga Ilmu Pengetahuan Indonesia. (2016) Jumlah
Usia Produktif Besar,
Indonesia
Berpeluang
Tingkatkan Produktivitas. Diakses pada tanggal 1
Agustus 2018
dari http://lipi.go.id/berita/jumlah-usia-
produktif-besar-indonesia-berpeluang-tingkatkan-
produktivitas/15220.
Manurung, R. V, Kurniawan, E. D., Hidayat, J., &
Risdian, C. (2012). Desain dan Fabrikasi Elektroda
Biosensor : Metode Teknologi Film Tebal. Jurnal
Ilmiah Elite Elektro, 3(1), 65–70.
Mariana, L., Andayani, Y., & Gunawan, R. (2013).
Analisis Senyawa Flavonoid Hasil Fraksinasi Ekstrak
Diklorometana Daun Keluwih (Artocarpus camansi),
6(2), 50–55.
Masuda T, Jitoe A, Mabry TJ. Isolation and structure
determination of cassumunarins A, B, C: new
antiinflammatory antioxidants from a tropical ginger,
Zingible cassumunar. J Am Oil Chem Soc.
1995;72:1053-57.
Misnadiarly. (2017). Rematik (1st ed.). Jakarta : Pustaka
Obor Popouler.
Muhtadi, Suhendi, A., W., N., & Sutrisna, E. (2013).
Potensi Daun Salam (Syzigium Polyanthum Walp.)
Dan Biji Jinten Hitam (Nigella Sativa Linn) Sebagai
Kandidat Obat Herbal Terstandar Asam Urat. Journal
of Chemical Information and Modeling, 53(9), 1689–
1699.
https://doi.org/10.1017/CBO9781107415324.004
Ningdyah, A. W., Alimuddin, A. H., & Jayuska, A.
(2015). Uji Toksisitas Dengan Metode BSLT (Brine
Shrimp Lethality Test) Terhadap Hasil Fraksinasi
Ekstrak Buah Tampoi (Baccaurea macrocarpa). Jurnal
Kimia Khatulistiwa, 4(1), 75–83.
Pandey, Amita and Tripathi, Shalini. (2014). Concept of
standardization, extraction and pre phytochemical
screening strategies for herbal drug. Journal of
Pharmacognosy and Phytochemistry. 2(5):115-119.
Parwata, I. M. O. A., Rita, W. S., & Yoga, R. (2009).
Isolasi dan uji antiradikal bebas minyak atsiri pada
daun sirih (Piper betle Linn) Secara Spektroskopi
Ultra Violet-Tampak. Jurnal Kimia, 3(1), 7–13.
Pokhrel, K., Yadav, BK., Jha, B., Parajuli, K., Pokharel,
RK. (2011). Estimation of Serum Uric Acid in Cases
of Hyperuricaemia and Gout. Journal of Nepal
Medical Association. 51(181):15-20.
Prasad, Sahdeo and Tyagi, K Amit. 2015. Ginger and Its
Constituent: Role in Prevention and Treatment of
Gastrointestinal Cancer. Gastroenterology Research
and Practice. http://dx.doi.org/10.1155/2015/142979
Quy Diem Do, Artik Elisa Angkawijaya, Phuong Lan
TranNguyen, Lien Huong Hyunh, Felycia Edi
Soetardjo, Suryadi ISmadji, Yi-Hsu Ju. (2014). Effect
of extraction solvent on total phenol content, total
flavonoid content, and antioxidant activity of
Limnophila aromatica. Journal of Food and Drug
Analysis. 22(30:296-302.
Rahman, H., Arifin, H., Dewi, G. K., & Rizal, Z. (2014).
Pengaruh Pemberian Jus Buah Sirsak (Annona
muricata, L) Terhadap Kadar Asam Urat Darah Mencit
Putih Jantan Hiperurisemia. In Perkembangan Terkini
Sains Farmasi dan Klinik IV (pp. 228–232). Padang.
Raji Y, Udoh US, Oluwadara OO, Akinsomisoye OS,
Awobajo O, Adeshoga K. Anti-Inflammatory and
Analgesic Properties of The Rhizome Extract of
Zingiber officinale. Departments of Physiology,
College of Medicine, Universitas of Ibadan.
Ravindran, P.N., Babu, K.N. 2005. Ginger The Genus
Zingiber. CRC Press : New York.
Rehman Riazur, Akram, M, Akhtar Naveed, Jabeen
Qaiser, Saeed Tariq, Shah, Ali MS, Ahmed Khalil,
Shaheen Ghazala, and Asif, MH. 2011. Zingiber
officinale Roscoe (pharmacological activity). Journal
of Medicinal Plants Research. 5(3): 344-348.
Sholihah, F. M. (2014). Diagnosis and Treatment Gout
Arthritis. J Majority, 3(7), 39–45.
Silva, De, O., G., Abeysundara, T., A., and Aponso, W.,
M., M. (2017). Extraction methods, qualitative and
quantitative technique for screening phytochemical
from plants. American Journal of Essential Oils and
Natural Products. 5(2):29-32.
Stoilova IA, Krastanov A, Stoyanova P Denev, Gargova S.
Antioxidant activity of a ginger extract (Zingiber
officinale). Food Chemistry. 2007;102:764–70.
Sruthi, D. R., and Indira, G. (2016). A comparative
evaluation of maceration, soxhlation and ultra sound
assisted extraction for the phytochemical screening of
the leaves of Nephelium lappaceum. L. Journal of
Pharmacognosy and Phytochemistry.5(5):386-389.
Suhendi, A., Nurcahyanti, Muhtadi, & Sutrisna, E. (2011).
Antihyperurisemia activity of water extract of black
seed (Coleus ambonicus Lour) in balb-c mice and its
standardization. Majalah Farmasi Indonesia, 22(2),
77–84.
Thayibah, R., Ariyanto, Y., & Ramani, A. (2018).
Hiperurisemia Pada Remaja di Wilayah Kerja
Puskesmas Arjasa Kabupaten Situbondo
Hyperuricemia in Adolescents ( 16-24 Years Old ) in
Arjasa Primary Health Center , Situbondo Regency,
6(1), 38–45.
Tahir, B., Saleh, C., & Pasaribu, S. P. (2013). Uji
Fitokimia, Toksisitas Dan Aktivitas Antioksidan
Alami Daun Tumbuhan Kelakai (Stenochlaena
The Effect of Ginger (Zingiber Officinale Roscoe) Fractionation in Decreasing Uric Acid Level of Hyperuricemic White Mice
473

palustris) Dengan Metode DPPH. In Prosiding
Seminar Nasional Kimia 2013 (pp. 141–146).
Tolistiawaty, I., Widjaja, J., Sumolang, P., Octaviani.
(2014). “Gambara Kesehatan pada Mencit di Instalasi
Hewan Coba”.Jurnal Vektor Penyakit. Sulawesi
Tengah.
Vogel, H. G. (2008). Drug Discovery and Evaluation:
Pharmacological Assays. Journal of Chemical
Information and Modeling (Third, Vol. 53). New
York: Springer-Verlag Berlin Heidelberg.
https://doi.org/10.1017/CBO9781107415324.004
Wahyuni, T., Widuri, A., Abdul, M., & Katrin. (2011).
“Uji Aktivitas Penghambatan Xantin Oksidase Ekstrak
Etanol 80% dari Tanaman Famili Combretaceae,
Lauraceae, Lythraceae, Oxalidaceae, Piperaceae,
Plumbaginaceae, dan
Smilacaceae,” 77–88.
Wall, G. (2010). Pharmacotherapy : Principles &
Practice. (M. A. Chisholm-Burns, B. G.Wells, T. l.
Schwinghammer, P. M.Malone, J. m. Kolesar, J. C.
Rotschafer, & J. t. Dipiro, Eds.). United States of
America: McGraw-Hill Companies, Inc.
https://doi.org/10.1036/0071448802
WHO. (2015). A Global Brief On Uric Acid. Geneva.
Wilmana, P.F. 2005. Farmakologi dan Terapi. Edisi ke-4.
Gaya Baru, Jakarta.
Yulion, R., Suhatri, & Arifin, H. (2017). Pengaruh Hasil
Fraksinasi Ekstrak Etanol Daun Lado-lado (Litsea
cubeba, Pers) Terhadap Kadar Asam Urat Serum
Darah Mencit Putih Jantan Tinggi Asam Urat. Jurnal
Sains Dan Teknologi Farmasi, 19(Desember).
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
474
