
Analysis of Whole Blood Quality: Number of Erythrocytes,
Leukocytes, Platelets, and pH Value during 28-day Storage
Serafica Btari Christiyani Kusumaningrum, Wiwit Sepvianti, Relita Pebrina, Yuni Andriyani
and Ana Nur Aini
Department of Blood Transfusion Technology, STIKES Guna Bangsa Yogyakarta, Jl. Ringroad Utara, Condongcatur,
Depok, Sleman, Yogyakarta, Indonesia
Keywords: Whole Blood, Erythrocytes, Leukocytes, Platelets.
Abstract: Whole blood contains all the elements of blood such as all blood cells, plasma, and clotting factors. It is used
in the treatment of massive bleeding. However whole blood has an expiration time limit. The quality of blood
decreases gradually due to storage time and causes blood cell lysis, so it directly affects blood cell counts and
pH. The aim of this study was to determine the quality of whole blood during 28day storage. Whole Blood
from blood bags containing CPDA-1 was used as a sample. The number of erythrocytes, leukocytes, and
platelets was measured by Hematology Analyzer. The results of the study showed that there was a decrease
in the number of erythrocytes from 5,02 x 10
6
cell/µL into 4,92x10
6
cell/µL. The number of leukocytes
decreased from 6,31x10
3
cell/µL to 3,17 x 10
3
cell/µL. Platelet count also decreased from 195x10
3
cell /µL to
81x10
3
cell/µL. The pH value decreased from 7.2 on to 6.9. This study concluded that there was a decrease in
the number of erythrocytes, leukocytes, platelets, and pH. The number of erythrocytes and pH was still
normal, while the number of leukocytes and platelets was below the normal range.
1
INTRODUCTION
Blood transfusion is a therapy to save someone’s
life using blood and its components (Booth and
Allard, 2017). The main principle of blood
transfusion is to safely and effectively replace
blood (Seghatchian et al., 2011). This way,
transfusion of various blood components is
required according to the indications in order to
reduce the risks of transfusion (Eldin and Teruya,
2012).
Whole blood is one of the blood components
that can be transfused when there is massive
bleeding (Avery and Avery, 2010). Whole blood
contains all the cellular components which include
erythrocytes, leukocytes, and platelets contained in
plasma (Hall et al, 2015). Whole blood can be
stored for 21 days at the temperature of 1-6°C in
anticoagulant citrate phosphate dextrose (CPD),
and can be stored for 35 days at 1-6°C in
anticoagulant citrate phosphate dextrose adenine
(CPDA-1) (Kurup et al., 2003; AABB, 2017). 450
ml of whole blood contains a 63 ml CPDA-1
anticoagulant solution; the amount of hemoglobin
is at least 45 grams per bag; the number of
leukocytes is <1 x 10
6
per bag and it is free from
bacterial contamination (
Kementerian Kesehatan
Republik Indonesia,
2015 and World Health
Organization, 2001).
An indication of administering whole blood to
replace red blood cells is when there is acute blood
loss with hypovolemia (WHO, 2001).
Unfortunately during storage, there are changes in
the structure and function of erythrocytes in whole
blood which can reduce the function and viability
of cells after transfusion (Kucukakin et al., 2011).
Besides, the storage period which is too long can
also decrease pH, DPG content, platelets and
coagulation factors in plasma (Hughes et al., 2007).
Thus, it is crucial to conduct a study of the quality
of whole blood by finding out the number of
erythrocytes, leukocytes, platelets, and pH during
the storage period of 28 days.
2
MATERIALS AND METHOD
Materials.
Hematology analyzer Sysmex XS-80i,
blood bank (temperature 2-6°C), blood collection
tubes were purchased from BD vacutainer.
Kusumaningrum, S., Sepvianti, W., Pebrina, R., Andriyani, Y. and Aini, A.
Analysis of Whole Blood Quality: Number of Erythrocytes, Leukocytes, Platelets, and pH Value during 28-day Storage.
DOI: 10.5220/0009592902610265
In Proceedings of the 1st International Conference on Health (ICOH 2019), pages 261-265
ISBN: 978-989-758-454-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
261
Sample.
One whole blood in CPDA-1 blood bag
was used as a sample.
Measurement of the number of erythrocytes,
leukocytes, and platelets.
The blood bag was
homogenized evenly by gently shaking the bag for
two minutes. The blood bag tube was cut so blood
could come out and 3mL of it was put into 28
sample tubes (red tubes). Prior to measurement, the
sample was left at room temperature for 1 minute
and then homogenized. The number of
erythrocytes, leukocytes, and platelets in the
sample tubes was counted using a hematology
analyzer, every day for 28 days.
Measurement of pH.
The blood samples in the
sample tubes were homogenized, then a pH
electrode was put into the samples until the
maximum line. The results obtained on the pH
meter were then read.
3 RESULTS AND DISCUSSION
Based on the results of this study, the pH and the
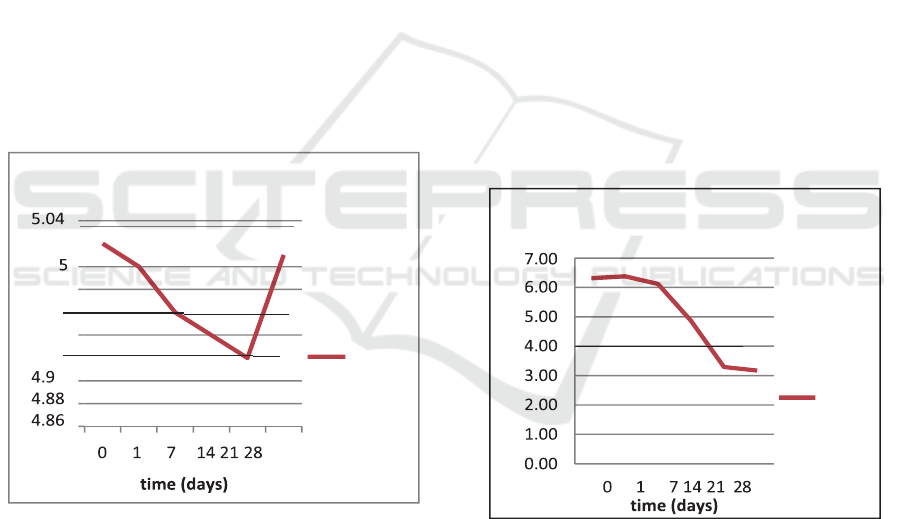
number of erythrocytes are as shown in Figure 1.
Figure 1: Number of erythrocytes on whole blood during
28-day storage.
Based on the results shown in Figure 1, the
number of erythrocytes declined gradually starting
from the baseline to the 21
st
day. On the baseline,
the number of erythrocytes was 5.02x10
6
cell/µL,
which declined on the 1
st
day to 5x10
6
cells/µL. On
the 21
st
day, the number of erythrocytes decreased
to 4.96x10
6
cells/µL on the 7
th
day, to
4.94x10
6
cells/µL. However, there was an increase
in the number of erythrocytes on the 28
th
day, i.e.
5.01x10
6
cell/µL.
A decrease in the number of erythrocytes from
the baseline to the 21
st
day is due to "storage
lesion", namely biochemical and biomechanical
changes in erythrocytes and storage media during
storage (Vani et al., 2015). This may change the
structure, function, and viability of erythrocytes
during the storage period (Kucukakin et al., 2011).
In addition, a factor that causes changes in the
viability of erythrocytes during storage is
glycolysis which continues to take place, resulting
in accumulation of metabolic waste which leads to
acidosis or a decrease in pH. Limited carbon source
in the blood bag can also slow down the rate of
glycolysis and the synthesis of energy in the form
of ATP, thus causing abnormal cell shape.
Unfortunately, the Hematology Analyzer could not
detect the presence of abnormal erythrocytes
(Zandecki et al., 2007), thus further observation of
cell shapes is necessary.
An increase in the number of erythrocytes on
the 28
th
day was believed to be due to uneven
homogenization during testing which caused cell
deposits on the bottom of the tubes. In fact, despite
a gradual decrease in the number of erythrocytes, it
is still within the normal range, i.e. 4.5 – 6.5 x 10
6
cell/µL (Riswanto, 2013).
Figure 2: Number of leukocytes on whole blood during
28-day storage.
Figure 2 presents the number of leukocytes in
whole blood during the 28-day storage. It can be
seen that there was a gradual decrease in the
number of leukocytes from the baseline to the 28th
day. On the baseline, the number of leukocytes
was6.31x10
3
/µL, which decreased to 6.38x10
3
/µL
on the 1st day. Finally, on the 28th day, the number
of leukocytes decreased to 3.17x10
3
/µL.
The decrease in the number of leukocytes could
occur in whole blood during storage. This is related
erythrocytes
5.02
4.98
5.02
5.01
5
4.96
4.96
4.94
4.94
4.92
4.92
erythocytes
number
leukocytes
6.31
6.38
6.11
4.86
3.29
3.17
leukocytes
number
cellcounts(x10
6
cell/µL)
cellcounts(x10
3
cell/µL)
ICOH 2019 - 1st International Conference on Health
262
to the functions of leukocytes, i.e. recognizing and
eliminating various foreign antigens such as
foreign proteins, viruses, and bacteria that enter the
body (Roenhorst et al., 1988). Thus, when there is
no antigen or viral infection in the whole blood bag
during storage, the number of leukocytes will
decrease.
Based on Regulation of
Kementerian
Kesehatan Republik Indonesia
No.91 tahun 2015
,
the number of leukocytes in each bag of
leukodepleted whole blood (whole blood of which
the number of leukocytes has been reduced) is
<1 x 106 per bag. This way, it can be said that the
number of leukocytes in the whole blood in this
study exceeded the normal range stipulated in this
regulation. This is because the whole blood used
was not leukodepleted whole blood so the number
of leukocytes had not been reduced using a filter.
According to Ampofo et al. (2002) and Seidl et
al. (1987), whole blood can be safely transfused if
screening for infectious diseases has been
performed and it is free from allogeneic
leukocytes. Allogeneic leukocytes can cause a
reaction in donor blood. The decrease in the
number of leukocytes in whole blood is very
important for blood recipients because it can
prevent the effects of leukocyte-mediated adverse
reactions such as alloimmunization, transfusion-
mediated cytomegalovirus (CMV) infections, and
non-hemolytic transfusion reactions (Dellinger and
Anaya, 2004; Vamvakas, 2006).
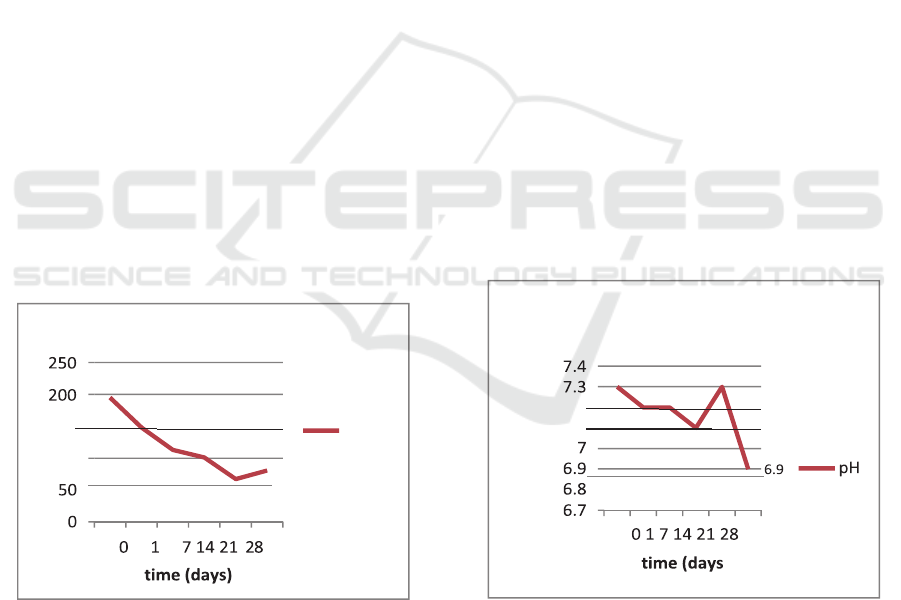
Figure 3: number of platelets on whole blood during 28- day
storage.
Platelets are cells that maintain vascular
integrity and play a role in hemostasis, i.e. in the
process of coagulation and prevention or stopping
bleeding. Platelets are produced from
megakaryocytes in the bone marrow and its life
span is around 8-10 days.
Based on the results of this study, the platelet
count in whole blood during the 28-day storage
decreased gradually from the baseline, i.e.
195x10
3
/µL, to 67x10
3
/µL on the 21st day
although there was a slight increase to 81x10
3
/µL
on the 28
th
day (Fig3). The normal platelet count in
the blood is 150,000-400,000/mm3. Thus it can be
said that the platelet count started to decrease on
the baseline from the normal range.
In general, a decrease in platelet count in whole
blood during storage is believed to be influenced
by hypoxia and anaerobic glycolysis due to oxygen
depletion. This makes the atmosphere in the blood
bag acidic, causing platelets to lose viability. In
addition, platelets should be stored at room
temperature because storage below 15
o
C may
change the structure of the platelet membrane
which also affects viability (Getz, 2019; Zucker
and Borreli, 1954).
Thus at present, regarding the use of platelets
for transfusion in blood clotting disorders, it is
recommended to use platelet concentrate
components stored in plasma. This is to reduce the
risk of platelets losing their viability after
transfusion due to the storage period.
However, previous studies showed that storing
platelets in whole blood by agitation up to 15 days
at a temperature of 4°C can maintain the functions
of platelets and can be used in traumatic wound
healing during surgery (Slichter et al., 2019).
Figure 4: number of platelets on whole blood during 28- day
storage.
The level of pH is a measure of the balance
between acidity and alkalinity in the body. The
normal pH of the blood is neutral to alkaline, i.e.
7.35 to 7.45. However, when the blood is outside
the body, i.e. in a blood bag, there are many factors
that cause changes in blood pH. This is what
platelets
195
150
148
100
113
101
67
81
platelets
number
pH
7.3 7.3
7.2 7.27.2
7.1 7.1
cellcounts(x10
3
cell/µL
pHvalue
Analysis of Whole Blood Quality: Number of Erythrocytes, Leukocytes, Platelets, and pH Value during 28-day Storage
263

underlies the measurement of blood pH as an
important part of the efforts to control the quality
of blood products. That is, at the end of storage, the
pH level of the blood in the blood bag may not be
≤ 6.4. In this study, the blood bag sample used
anticoagulant Citrate Phosphate Dextrose Adenine
(CPDA-1) of which the ratio between blood
volume and anticoagulant volume was x: x. During
the 28 day storage, the blood pH was measured on
days 0, 1, 7, 14, 21 and 28 days. According to the
measurement done 6 hours after tapping (day 0),
the blood pH was
7.30. On the 1st day, the pH level was observed to
decrease by 0.1 to 7.20 and this remained stable
until the 7th day. However, there was another pH
decline on the 14th day by 0.2 to 7.10. On the last
storage day, i.e. the 28
th
day, there was a quite
significant decrease in the pH, i.e. 6.90. Some of
the factors that might have an important effect on
lowering the pH level during storage are:
1.
The use of anticoagulant CPDA-1 which
contains an acid compound
2.
Respiration by blood cells produces carbon
dioxide. In the body, carbon dioxide is
processed by the lungs, but the blood bag
does not have such function, causing
respiratory acidosis which disrupts blood
buffer retention, thus lowering pH level.
3.
Cellular metabolism which continues to take
place during storage, decreasing the ATP
level and glucose in the blood. One of the
products of cellular metabolism is lactic acid.
Lactic acid accumulation could also lower
the pH level.
4 CONCLUSIONS
Based on the results of this study, in general, the
pH value and the number of erythrocytes,
leukocytes, and platelets decrease during the 28-
day storage. In fact, the number of erythrocytes and
pH values are still within the normal range, while
the number of leukocytes and platelets is below the
normal range. Therefore, it is important to process
blood into particular blood components to
guarantee and provide the quality of the
components needed.
REFERENCES
AABB. 2017. Circular of information for the use of Human
Blood and Blood Components. Armed Services Blood
Program
Ampofo, W., Trebi, N. N., Ansah, J., Abe, K.
2002.Prevalence of blood-borne infectious diseases in
blood donors in Ghana. J. Clin Microbiol, 40, 3523–
3525.
Avery DM, Avery KT. 2011. Blood component therapy.
Am J Clin Med, 7:57-9.
Booth, C and Allard, S. 2017. Blood transfusion. Medicine,
45(4): 244-250.
Dellinger, E. P., Anaya, D. A., 2004. Infectious and
immunologic consequences of blood transfusion. Crit.
Care, 8, 18–23.
Eldin KW, dan Teruya J. 2012. Blood components for
hemostasis. Lab Med, 43; 237-44.
Getz TM. 2019. Physiology of cold platelets. Transfus
Apher Sci, 58(1): 12-15.
Hall JE, Guyton and Hall. 2015. Textbook of Medical
Physiology. 13th edition, Saunders
Hughes, J.D., Macdonald, VW, Hess, J.R. 2007.Warm
storage of whole blood for 72 hours. Transfusion,
47(11)
Kementerian Kesehatan Republik Indonesia. 2015.
Peraturan Menteri Kesehatan No.91 Tahun 2015
tentang Standar Pelayanan Transfusi Darah. Jakarta
Kucukakin B, Kocak V, Lykkesfeldt J, Nielsen HJ,
Magnussen K, Rosenberg J, Gogenur I. 2011. Storage-
induced increase in biomarkers of oxidative stress and
inflammation in red blood cell components. Scand J
Clin Lab Invest, 71:299-303.
Kurup, PA., Arun, P., Gayathri, NS., Dhanya, CR., Indu R.
2003. Modified fomulation of CPDA for storage of
whole blood and of SAGM for storage of red blood
cells, to maintain the concentration of 2,3-
diphosphoglycerate.Vox Sanguinis, 85(4):253-61
Riswanto, 2013. Pemeriksaan Laboratorium Hematologi.
Alfamedia & Kanal Medika. Yogyakarta
Roenhorst, H. W., Kallenberg, G. M., The, T. H., 1988. The
cellular immune response to cell-associated and cell-
free cytomegalovirus (CMV) antigens after primary
CMV-infection in non-immunocompromised hosts:
Development and maintenance of CMV-latency and its
influence on immunocompetence. Clin. Exp. Immunol,
74, 326–332
Seidl, S.,Kuhnl, P.,1987. Transmission of diseases by blood
transfusion. World J. Surg. 11, 30–35
Seghatchian, J. Hervig T., Putter JS. 2011. Effect of
pathogen inactivation on the storage lesion in red cells
and platelet concentrates. Transfus Apher Sci, 45:75- 94
Slichter, S. J., Fitzpatrick, L., Osborne, B., Christoffel,
T.,Gettinge, I., Pellham, E. Bailey, L., Jones, MK.,
Herzig, MC., Cap, A. 2019. Platelets stored in whole
blood at 4°C: in vivo posttransfusion recoveries and
survivals and in vitro hemostatic function. Transfusion,
00:1-9
Vamvakas, E. C., 2006. The case against universal white
blood cell reduction. ISBT Sci. Series, 1, 64–72.
ICOH 2019 - 1st International Conference on Health
264

Vani R., Suomya, R., Manasa, K, Carl, H. 2015. Storage
lesions in blood components. Oxidants and
Antioxidants in Medical Science, 4(3):125-132
World Health Organization. 2001. 2001. The Clinical use
of blood: handbook. World Health Organization.
Geneva.
Zandecki, M., Genevieve, F., Gerald, J., Gordon, A. 2007.
Spurious counts and spurious results on hematology
analyzers. International Journal of Laboratory
Hematology
Zucker, MB and Borrelli, J. 1954. Reversible alterations in
platelet morphology produced by anticoagulants and by
cold, Blood, 9:602-608.
Analysis of Whole Blood Quality: Number of Erythrocytes, Leukocytes, Platelets, and pH Value during 28-day Storage
265
