
Antioxidant and Anti-inflammatory Activity of Salacca zalacca
(Gaertn.) Voss Peel Ethanolic Extract
on Lead Induced Fibroblast Cells
Ermi Girsang
1
, I Nyoman Ehrich Lister
1
, Chrismis Novalinda Ginting
1
, Sri Lestari Nasution
1
,
Suhartina Suhartina
1
, Ubaydillah Zedd Munshy
2
, Rizal Rizal
2
, Wahyu Widowati
3
1
Universitas Prima Indonesia, Jl. Belanga No. 1, Medan 20118, North Sumatera, Indonesia
2
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Jl. Babakan Jeruk II No. 9, Bandung 40163, West
Java, Indonesia
3
Faculty of Medicine, Maranatha Christian University, Jl. Surya Sumantri No. 65, Bandung 40164, West Java, Indonesia
Keywords: Lead, intracellular ROS, Cytotoxic, Salacca zalacca.
Abstract: Lead toxicity is a serious environmental disease and its effects on the human body are overwhelming. Lead
can increase reactive oxygen species (ROS) levels in the body which results in oxidative stress. Elevated ROS
levels can stimulate inflammation and cellular aging. Plants extract have the abilities as antioxidant and anti-
inflammatory agent to prevent aging and toxicity including Salacca zalacca peels extract (SPE). Cytotoxicity
assay of SPE towards fibroblast cells (BJ) was handle using MTS (3-4,5-dimethylthiazol-2-yl)-5-(3-
carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium). Intracellular ROS levels were observed by flow
cytometry using DCF-DA fluorescent probe. Fibroblast cells were incubated at 37
0
C, 5% CO
2
, treated by 25
and 100 µg/ml SPE for 4 hours and followed by 400 µM Pb for 72 hours. Anti-inflammatory capacity was
conducted using ELISA to measure IL-10 and TNF-α. SPE at 3.13-100 µg/ml were nontoxic to the BJ cells.
Accumulation of intracellular ROS levels in lead-induced BJ cells were decreased by treatment using SPE at
25 and 100 µg/ml. SPE at 25 and 100 µg/ml elevated IL-10 and decreased TNF-α related to positive control
(lead-induced cells). This research shows that S. zalacca peels extract has the ability as protective effect
related to Pb poisoning.
1 INTRODUCTION
Lead (Pb) toxicity is a serious environmental disease
and its effects on the human body are overwhelming
(Wani et al., 2015). Lead can cause an increase in
reactive oxygen species (ROS) levels in the body
which results in oxidative stress. Oxidative stress is a
condition where there is an inequality between the
antioxidant defences and level of ROS, and causes
oxidative damage (Redza-Dutordoir and Averill-
Bates, 2016). The generation of ROS in cells is in
equilibrium with the defence system against free
radicals. Excessive formation of ROS can cause stress
response which then causes an increase in the aging
process of cells (Kuilman et al., 2010). Conversely,
low ROS levels affect the lifetime of an organism
(Davalli et al., 2016). Several disease and aging
process affected by accumulation of ROS which
induces apoptosis and can drive even skin cancer
(Widowati et al., 2016). Furthermore, ROS
production have a crucial role to the elevate of many
inflammatory disorder (Mittal et al., 2014).
Salacca zalacca known as snake fruit is a plant
species of the palm tree family (Arecaceae) that
native to Indonesia. Snake fruit peel are the major
waste of the consumption because is hard and
inedible, even so the previous study discover that
snake fruit peel contains important phenolic
compound such as rutin, chlorogenic acid, caffeic
acid, and protocatechuic acid (Girsang et al., 2019).
Moreover, phytochemical compounds discovered in
snake fruit peel were known to be active as an
antioxidant (Gulcin, 2006; Kikuzaki et al., 2002;
Liang and Kitts, 2015; Yang et al., 2008). In this
study, we investigated the antioxidant and anti-
inflammatory effects of SPE on lead-induced
fibroblast cells (BJ) as aging role model.
68
Girsang, E., Lister, I., Ginting, C., Nasution, S., Suhartina, S., Munshy, U., Rizal, R. and Widowati, W.
Antioxidant and Anti-inflammatory Activity of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead Induced Fibroblast Cells.
DOI: 10.5220/0009588200680073
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 68-73
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1 Cytotoxicity Assay
Human fibroblast cell line (BJ) (ATCC
®
CRL-
2522
TM
) was received from Aretha Medika Utama,
Biomolecular and Biomedical Research Center,
Bandung, Indonesia. We detected to determine the
maximum tolerance concentration of SPE on BJ cells
and to determine the optimal oxidative damage
concentration of lead (Pb) for the following
experiments. BJ Cells were cultured in MEM
(Biowest, L0416-500) suplemented with 10% fetal
bovine serum (FBS) (Biowest, S1810-500), 1%
Antibiotic/antimycotic (ABAM, Biowest,
L0010100), 1% Nanomycopulitine (Biowest, L-X16-
100), 1% Amphotericin B (Gibco, 1%), 0.1%
Gentamicin (Gibco, 15750045). Cells were incubated
at 37
0
C in a humidified atmosphere with 5% CO
2
.
After that, 80% of cells confluency, 5 x 10
3
cells were
seeded in each well of 96-well plate. After 24 h
incubation, the cells were treated with SPE at various
concentrations (3.13, 6.25, 12.5, 25, 50, and 100
µg/ml) for 24 h. To elect cell viability, 3-(4,5-
dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-
2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay
(Promega, Madison, WI, USA) was used. MTS was
added to each well at a ratio of 1:10 (Novilla et al.,
2017; Widowati et al., 2016) The plate was incubated
in 5% CO
2
at 37
0
C for 4 h. Absorbance was measured
at 490 nm on a microplate reader. The data are given
as the percentage of viable cells (%) and data were
analyzed using ANOVA and continued by Tukey post
hoc test.
2.2 Intracellular Reactive Oxygen
Species Analysis
The intracellular ROS levels were detected by flow
cytometry using a DCF-DA fluorescent probe
(invitrogen) in accordance with the method of Jie et
al (Jie et al., 2006; Prahastuti et al., 2019; Widowati
et al., 2014) with slight modification. After 7 days of
culture, BJ cells were digested with trypsin-EDTA
and 10
5
cells were incubated with 10 µM DCF-DA at
37
0
C for 30 min, after that incubated with SPE (25
and 100 µg/ml) for 4 h and followed by 400 µM Pb
for 3 days. The intracellular ROS levels were
analyzed using Milteny Flow Cytometer (MAQS
quant). BJ Cells treated with Pb without SPE
treatment showed as controls. The analyzed
fluorescence values were expressed as a percentage
of control.
2.3 IL-10 and TNF-α Evaluation
Evaluation of Interleukin 10 (IL-10) and Tumour
necrosis factor-α (TNF-α) were conducted using
ELISA Kit, IL-10 (Elabsci, E-EL-H0103) and TNF-α
(Elabsci, E-EL-H0109), conditioned medium (CM)
was used as a sample. Conditioned medium was
collected after culture of fibroblast cells and treatment
using SPE (25 and 100 µg/ml for 4 hours) following
by inducted using Pb (400 µM for 72 hours). Cells
were incubated at 37
0
C in a humidified atmosphere
with 5% CO
2
. The method was in accordance with
manufacturer protocol. Sample absorbances were
read at 450 nm using spectrophotometer (Multiskan
GO, Thermo Scientific). Colour changes of samples
are evaluated then read immediately at 450 nm
wavelength and the IL-10, TNF-α concentration can
be determined based on a protein standard curve
(Noverina et al., 2019; Widowati et al., 2018, 2017).
3 RESULTS AND DISCUSSION
Skin aging is a perplexing natural phenomenon
defined by continuous loss of structural stability and
function of the skin, beside skin aging can be induced
by environmental factors such as lead exposure.
Continuous lead exposure can lead to increased
physical changes in the skin and connective tissue
over intracellular ROS and cell contents (Widowati et
al., 2016).
3.1 Cytotoxicity Assay
Many cell biological studies using fibroblast for
standard cell line. Fibroblasts are liable for the
metabolism and synthesis of most connective-tissue
components and also play an effective part in the
body’s natural immune responses. Fibroblasts are the
major cells in granulation tissue and scar forming
over inflammation. In this research we define the
cytotoxicity of SPE toward fibroblast cells, depend on
the results SPE decreased cell growth only at the
highest concentration (100 µg/ml).
To employ this natural compound to health
concern, toxicity assay was conducted to assure
safety. Tetrazolium compound 3-(4,5-
dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-
2-(4-sulfophenyl)-2H-tetrazolium (MTS) has been
used for this assay. The MTS compound is bio-
reduced by cells into a colored formazan product due
to conversion by dehydrogenase enzymes in
metabolically active cells (Novilla et al., 2017).
Antioxidant and Anti-inflammatory Activity of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead Induced Fibroblast Cells
69
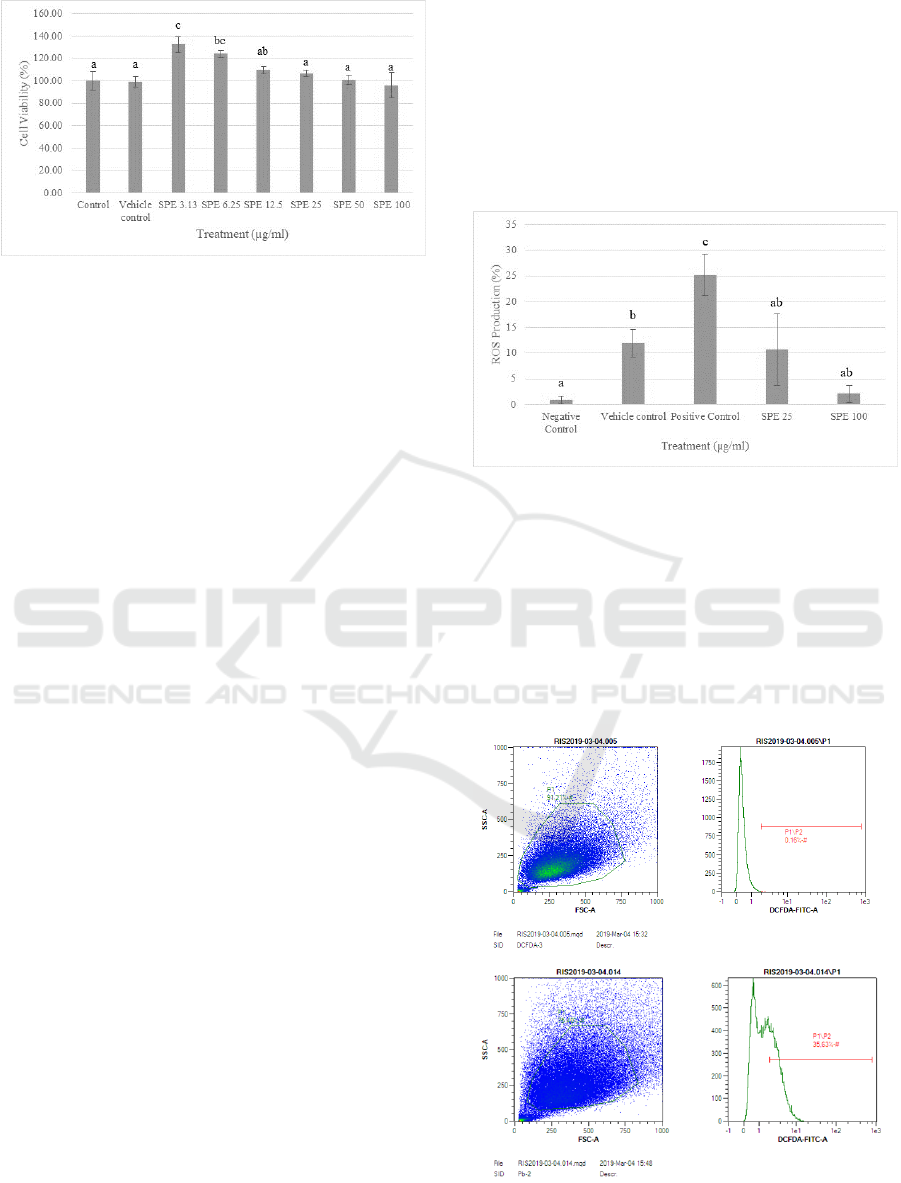
Figure 1: Effect of S. zalacca peels ethanolic extract (SPE)
on cytotoxicity of fibroblast cells. *The histograms are
presented as mean ± standard deviation. The data were
analyzed with ANOVA and continued with Tukey post hoc
test. Different letters (a, ab, bc, c) indicate significant
differences among treatment. Control: cells without any
treatments; Vehicle Control: cells with DMSO 10%
treatment.
The total of each concentration of SPE were
found non-cytotoxic and safe for fibroblast cell (3.13,
6.25, 12.5, 25, 50, and 100 µg/ml) (Figure 1), as the
viability of the cells was more than 75%
(Kanlayavattanakul et al., 2012). The lower number
of cell viability was obtained from the highest
concentration of SPE but not cytotoxic. The data of
viable cell number were presented in percentage of
viable cell. Depend on the post hoc test results, the
safe concentration that can be used for further
experiments and not harmful for cells are found in
SPE concentrations 25 and 100 µg/ml.
3.2 Intracellular ROS Analysis
The accretion of ROS which generate apoptosis is
then an main contributor to several disorders and
aging (Orr and Sohal, 1994). The accretion of
intracellular ROS in aging process can drives to loss
of skin elasticity and cause formation of wrinkle,
brown spots, uneven pigmentation, and even skin
cancer (Widowati et al., 2016). Our outcome in
accordance with previous research which express that
lead exposure can elevate the ROS levels (Lopes et
al., 2016). Salacca zalacca peel extract has natural
compound such as chlorogenic acid (Liang and Kitts,
2015). Addition of chlorogenic acid can decrease
ROS levels, in accordance with Hoelzl et al (Hoelzl
et al., 2010), chlorogenic acid can decrease levels of
ROS approximately 20.3% after hydrogen peroxide
exposure.
Fluorescence intensity used as an indicator of
ROS production levels. Figure 2 shows significant
increase of ROS levels (relatively 25%) in cells
induced lead (400 µM) for 72 hours compared to
negative control (Cells stained using DCF-DA). The
outcome of treatment using SPE with concentration
of 25 and 100 µg/ml can significantly decrease ROS
levels (relatively 15% and 23%). The better optimal
SPE concentration in decreasing ROS levels was 100
µg/ml, but the concentration did not contrast
significantly from SPE concentration of 100 µg/ml
based on Tukey post hoc test.
Figure 2: Effect SPE toward ROS production on lead-
poisoned fibroblast cell.
The histograms are presented as
mean ± standard deviation. The data were analyzed with
ANOVA and continued with Tukey post hoc test (p<0.05).
Different letters (a, ab, b, c) indicate significant differences
among treatment. Negative control: cells without any
treatments; Vehicle control: cells with DMSO 10%
treatment; Positive control: cells with Pb induced; SPE 25:
cells treated Pb + SPE 25 µg/ml treatment; SPE 100: cells
treated Pb + SPE 100 µg/ml treatment.
a
b
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
70
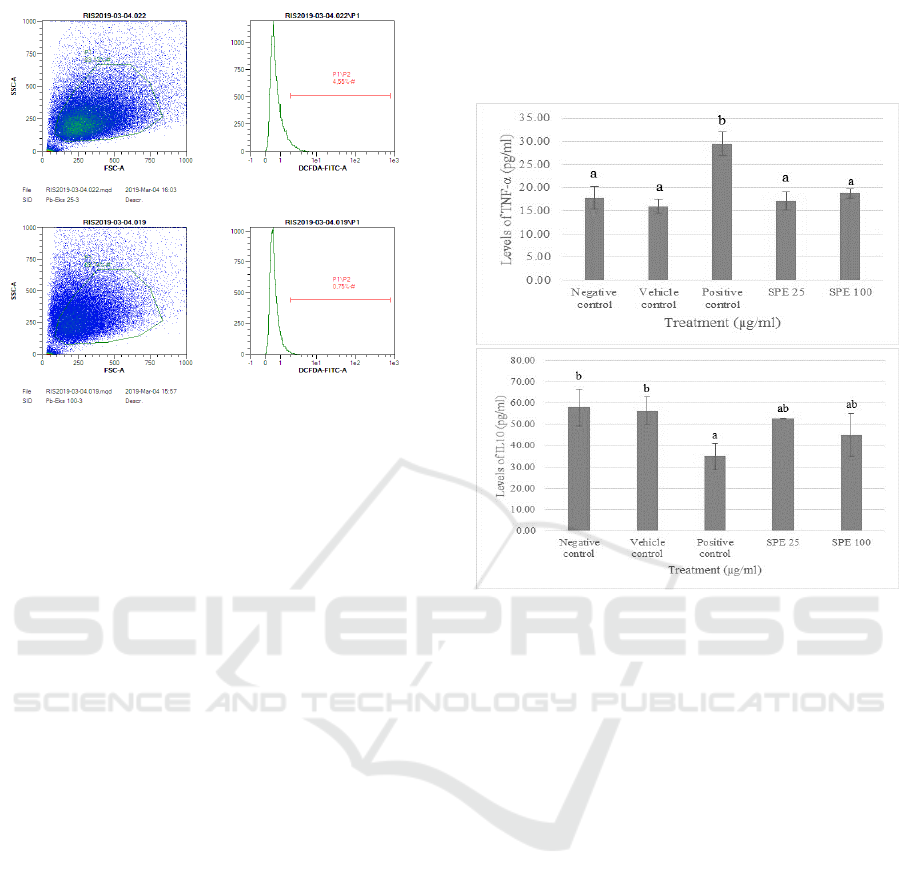
Figure 3: The representative of dot blot of various
concentrations of SPE treatment on lead-poisoned
fibroblast cell toward ROS level. TBHP = tert-butyl
hydroperoxide, DCFDA = 2’,7’-dichlorofluorescin
diacetate. a: Negative control (Cell only + DCFDA)
(0.16%); b: Positive control (Cell only + Pb) (35.63%); c:
S. zalacca Peels Extract induced 25 µg/ml (4.55%); d: S.
zalacca Peels Extract induced 100 µg/ml (0.75%).
3.3 IL-10 and TNF-α Evaluation
Accumulation of intracellular ROS production can
improve an increase the expression of NF-kB, leading
to the upregulation of factors elaborated in
inflammation. Tumour Necrosis Factor-α (TNF-α)
effective releasers of IL-6 which is one of the basic
cytokines to be related to the aging process (Morley
and Baumgartner, 2011). The effect of TNF-α can
turn triggers effects that elevate inflammation that can
indicate with elevated ROS levels (B. Marcu et al.,
2010). Contrary, IL-10 is a cytokine which has a role
as an anti-inflammatory and manage by stimulating
antagonist proteins against TNF-α as pro-
inflammatory cytokine (Wojdasiewicz et al., 2014).
During inflammation, IL-10 and TNF-α are
cytokines that responsible. TNF-α and IL-10 has
contrary role, TNF-α has a role as pro-inflammatory
cytokine (Laksmitawati et al., 2016), while IL-10 has
a role of anti-inflammatory cytokine (Morley and
Baumgartner, 2011). Evaluation of IL-10 and TNF-α
have been conducted using ELISA method.
Fibroblast cells induced with lead has the highest
levels of TNF-α among others, although SPE
inclusion shows significant decrease in TNF-α.
Besides, fibroblast cells lead-induced has the lowest
levels of IL-10 and inclusion of SPE can elevate the
IL-10 levels. SPE at 25 and 100 µg/ml can reduce the
TNF-α levels but not significant, as well as IL-10
levels, both SPE concentration can elevate IL-10
levels but the results are not significant based on post
hoc test.
Figure 4: Effect of SPE toward TNF-α level (a) and IL-
10 level (b) on lead-poisoned cells model. The
histograms are presented as mean ± standard deviation. The
data were analyzed with ANOVA and continued with
Tukey post hoc test (P<0.05). Different letters (a, b) on
figure A and (a, ab, b) on figure B indicate significant
differences among treatment.
Negative control: cells
without any treatments; Vehicle Control: cells with DMSO
10% treatment; Positive control: cells with Pb induced; SPE
25: cells treated Pb + SPE 25 µg/ml treatment; SPE 100:
cells treated Pb + SPE 100 µg/ml treatment.
4 CONCLUSIONS
This current study exhibit the compatibility of S.
zalacca peels ethanolic extract as a promising
antioxidant and anti-inflammatory agent through
decrease of intracellular ROS levels and anti-
inflammatory abilities to decrease pro-inflammatory
cytokine (TNF-α) and increase anti-inflammatory
cytokine (IL-10) on lead-poisoned human fibroblast
cells.
d
c
a
b
Antioxidant and Anti-inflammatory Activity of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead Induced Fibroblast Cells
71

ACKNOWLEDGEMENTS
We are gratefully acknowledge the financial support
of the Research Center and Service Community,
Universitas Prima Indonesia, Medan, North
Sumatera, Indonesia for research grant 2018. This
study also was funded, facilitated and supported by
Biomolecular and Biomedical Research Center,
Aretha Medika Utama, Bandung, West Java,
Indonesia. We are thankful to Hanna Sari Widya
Kusuma, Ika Adhani Sholihah, Dewani Tediana
Yusepany, Dwi Surya Artie, Anisa Siwianti from
Biomolecular and Biomedical Research Center,
Aretha Medika Utama, Bandung, West Java,
Indonesia for their valuable assistance.
REFERENCES
Marcu, B.K., Otero, M., Olivotto, E., Maria Borzi, R., B.
Goldring, M. (2010). 'NF-kB signaling: multiple angles
to target OA'. Curr. Drug Targets. 11(5), 599–613.
Davalli, P., Mitic, T., Caporali, A., Lauriola, A., D’Arca, D.
(2016). 'ROS, Cell Senescence, and Novel Molecular
Mechanisms in Aging and Age-Related Diseases'.
Oxid. Med. Cell. Longev. 2016, 1–18.
Girsang, E., Ginting, C.N., Nyoman, I., Lister, E.,
Widowati, W., Haryo, S., Wibowo, B., Perdana, F.S.,
Rizal, R. (2019). 'In silico analysis of phytochemical
compound found in snake fruit (Salacca zalacca) peel
as anti-aging agent'. Thai J. Pharm. Sci. 43(2), 105–
109.
Gulcin, I. (2006). 'Antioxidant activity of caffeic acid (3,4-
dihydroxycinnamic acid)'. Toxicology. 217(2-3), 213–
220.
Hoelzl, C., Knasmüller, S., Wagner, K.-H., Elbling, L.,
Huber, W., Kager, N., Ferk, F., Ehrlich, V., Nersesyan,
A., Neubauer, O., Desmarchelier, A., Marin-Kuan, M.,
Delatour, T., Verguet, C., Bezençon, C., Besson, A.,
Grathwohl, D., Simic, T., Kundi, M., Schilter, B.,
Cavin, C. (2010). 'Instant coffee with high chlorogenic
acid levels protects humans against oxidative damage
of macromolecules'. Mol. Nutr. Food Res. 54(12),
1722–1733.
Jie, G., Lin, Z., Zhang, L., Lv, H., He, P., Zhao, B. (2006).
'Free Radical Scavenging Effect of Pu-erh Tea Extracts
and Their Protective Effect on Oxidative Damage in
Human Fibroblast Cells'. J. Agric. Food Chem. 54(21),
8058–8064.
Kanlayavattanakul, M., Ospondpant, D., Ruktanonchai, U.,
Lourith, N. (2012). 'Biological activity assessment and
phenolic compounds characterization from the fruit
pericarp of Litchi chinensis for cosmetic applications'.
Pharm. Biol. 50(11), 1384–1390.
Kikuzaki, H., Hisamoto, M., Hirose, K., Akiyama, K.,
Taniguchi, H. (2002). 'Antioxidant Properties of Ferulic
Acid and Its Related Compounds'. i. 50(7), 2161–2168.
Kuilman, T., Michaloglou, C., Mooi, W.J., Peeper, D.S.
(2010). 'The essence of senescence'. Genes Dev. 24(22),
2463–2479.
Laksmitawati, D.R., Prasanti, A.P., Larasinta, N., Syauta,
G.A., Hilda, R., Ramadaniati, H.U., Widyastuti, A.,
Karami, N., Afni, M., Rihibiha, D.D., Kusuma, H.S.W.,
Widowati, W. (2016). 'Anti-Inflammatory Potential of
Gandarusa (Gendarussa vulgaris Nees) and Soursoup
(Annona muricata L) Extracts in LPS Stimulated-
Macrophage Cell (RAW264.7)'. J. Nat. Remedies.
16(2), 73.
Liang, N., Kitts, D. (2015). 'Role of Chlorogenic Acids in
Controlling Oxidative and Inflammatory Stress
Conditions'. Nutrients. 8(1), 16.
Lopes, A.C.B.A., Peixe, T.S., Mesas, A.E., Paoliello,
M.M.B. (2016). 'Lead Exposure and Oxidative Stress:
A Systematic Review'. pp. 193–238.
Mittal, M., Siddiqui, M.R., Tran, K., Reddy, S.P., Malik,
A.B. (2014). 'Reactive Oxygen Species in Inflammation
and Tissue Injury'. Antioxid. Redox Signal. 20(7),
1126–1167.
Morley, J.E., Baumgartner, R.N. (2011). 'Cytokine-Related
Aging Process. Journals Gerontol'. Ser. A Biol. Sci.
Med. Sci. 59(9), M924–M929.
Noverina, R., Widowati, W., Ayuningtyas, W., Kurniawan,
D., Afifah, E., Laksmitawati, D.R., Rinendyaputri, R.,
Rilianawati, R., Faried, A., Bachtiar, I., Wirakusumah,
F.F. (2019). 'Growth factors profile in conditioned
medium human adipose tissue-derived mesenchymal
stem cells (CM-hATMSCs)'. Clin. Nutr. Exp. 24, 34–
44.
Novilla, A., Djamhuri, D.S., Nurhayati, B., Rihibiha, D.D.,
Afifah, E., Widowati, W. (2017). 'Anti-inflammatory
properties of oolong tea ( Camellia sinensis ) ethanol
extract and epigallocatechin gallate in LPS-induced
RAW 264.7 cells'. Asian Pac. J. Trop. Biomed. 7(11),
1005–1009.
Orr, W., Sohal, R. (1994). 'Extension of life-span by
overexpression of superoxide dismutase and catalase in
Drosophila melanogaster'. Science. 263(5150), 1128–
1130.
Prahastuti, S., Hidayat, M., Hasiana, S.T., Widowati, W.,
Amalia, A., Qodariah, R.L., Rizal, R., Kusuma,
H.S.W., Khoiriyah, Z. (2019). 'Ethanol Extract of Jati
Belanda (Guazuma ulmifolia L.) as Therapy for
Chronic Kidney Disease in In Vitro Model'. J. Reports
Pharm. Sci. 8, 299-235.
Redza-Dutordoir, M., Averill-Bates, D.A. (2016).
'Activation of apoptosis signalling pathways by reactive
oxygen species'. Biochim. Biophys. Acta - Mol. Cell
Res. 1863(12), 2977–2992.
Wani, A.L., Ara, A., Usmani, J.A. (2015). 'Lead toxicity: a
review'. Interdiscip. Toxicol. 8(2), 55–64.
Widowati, W., Afifah, E., Mozef, T., Sandra, F., Rizal, R.,
Amalia, A., Arinta, Y., Bachtiar, I., Murti, H. (2018).
'Effects of insulin-like growth factor-induced wharton
jelly mesenchymal stem cells toward chondrogenesis in
an osteoarthritis model'. Iran. J. Basic Med. Sci. 21(7),
745–752.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
72

Widowati, W., Widyanto R.M., Husin, W., Ratnawati, H.
Laksmitawati, D.R. Setiawan, B., Nugrahenny, D.,
Bachtiar, I. . (2014). ' Green tea extract protects
endothelial progenitor cells from oxidative insult
through reduction of intracellular reactive oxygen
species activity'. Irannian J Basci Med Sci. 17, 702–
709.
Widowati, W., Fauziah, N., Herdiman, H., Afni, M., Afifah,
E., Kusuma, H.S.W., Nufus, H., Arumwardana, S.,
Rihibiha, D.D. (2016). 'Antioxidant and Anti Aging
Assays of Oryza sativa Extracts, Vanillin and Coumaric
Acid'. J. Nat. Remedies. 16(3), 88-99
Widowati, W., Widyastuti, H., Murti, H., Laksmitawati, D.,
Sari, H., Kusuma, W., Rizal, R., Afifah, E., Sumitro, S.,
Widodo, M., Bachtiar, I. (2017). 'Interleukins and
VEGF secretome of human wharton’s Jelly
mesenchymal stem cells-conditioned medium
(hwjmscs-CM) in different passages and oxygen
tensions'. Biosci. Res. 14, 776-787.
Wojdasiewicz, P., Poniatowski, Ł.A., Szukiewicz, D.
(2014). 'The Role of Inflammatory and Anti-
Inflammatory Cytokines in the Pathogenesis of
Osteoarthritis'. Mediators Inflamm. 2014, 1–19.
Yang, J., Guo, J., Yuan, J. (2008). 'In vitro antioxidant
properties of rutin'. LWT - Food Sci. Technol. 41(6),
1060–1066.
Antioxidant and Anti-inflammatory Activity of Salacca zalacca (Gaertn.) Voss Peel Ethanolic Extract on Lead Induced Fibroblast Cells
73
