
In-vivo Test of Chlorella Protein Fragments as Nucleotide Vaccine
Candidates in Grouper Viral Nervous Necrosis (VNN) Infection
against Haematological Response
Uun Yanuhar
*
, Muhammad Musa, Diana Arfiati, Nico Rahman Caesar and Nur Sakinah Junirahma
Faculty of Fisheries and Marine Science, Brawijaya University, Indonesia
Keywords: Humpback grouper, Chlorella vulgaris, VNN, Haematological.
Abstract: Grouper (Cromileptes altivelis) is a species of fish with important economic values both in the national and
international markets. The disease that has been reported by researchers is Viral Nervous Necrosis (VNN)
which can cause mass death in groupers, especially in larval and juvenile stadia. Based on the problems, a
research is needed on haematological analysis of groupers (Cromileptes altivelis) infected with Viral
Nervous Necrosis by in-vivo testing using protein fragments of C. vulgaris. This research employed an
experiment method using 5 treatments, namely (A) healthy fish, (B) VNN-infected fish, (C) VNN-infected
fish with administration of C.vulgaris crude extracts of 17 µg mL
-1
, (D) VNN-infected fish with
administration of C.vulgaris crude extract of 33 µg mL
-1
, and (E) VNN-infected fish with administration of
C.vulgaris crude extract of 50 µg mL
-1
. Observations of haematological parameters included erythrocytes,
leukocytes, haemoglobin, and haematocrit. The observation results showed an erythrocyte value of 97 x 104
cells/mm
3
in treatment (C), 107 x 104 cells/mm
3
in treatment (D), and 94 x 104 cells/mm
3
in treatment (E).
The observation results of leukocyte values were 150,000 cells/mm
3
in treatment (C), 133,300 cells/mm
3
in
treatment (D), and 139,000 cells/mm
3
in treatment (E). Furthermore, the observation results of haemoglobin
showed a value of 5 gr/100 ml in treatment (C), 6 gr/100 ml in treatment (D), and 5 gr/100 ml in treatment
(E). As for the haematocrit parameter, the results obtained from the observation were 18% in treatment (C),
22% in treatment (D), and 15% in treatment (E). Based on this research, the haematological status of VNN-
infected groupers was not good. However, the results of the in-vivo testing conducted showed that
administration of C. vulgaris extract gave a positive result on improving the haematological status of
groupers (C. altivelis) infected with VNN with the optimal dose of 33 µg mL
-1
.
1 INTRODUCTION
One of the potential of sea waters that has been
developed and is starting to show a growth in
international market is grouper. Grouper is widely
distributed in waters that are inhabited by coral in
tropical and subtropical regions. Some types of
grouper that have been targeted in the market are the
duck grouper (Cromileptes altivelis), tiger grouper
(Epinephelus fuscoguttatus), leopard grouper
(Epinephelus leopardus) and mud grouper
(Epinephelus coioides). These types of grouper have
a high selling value. In addition, its cultivation
process only need and use local components.
(Sudaryatma et al., 2012). However, the hybrid of
cantang grouper is experiencing a decrease in
production due to some environmental stresses, for
example, poor water quality, which makes the
cantang grouper is susceptible to viral, bacterial,
stressful infections from time to time resulting in
poor growth and ultimately death. (Noor et al., 2018).
The obstacle of cultivation in the Epinephelus
group (Grouper) in Indonesia is the limited supply of
fish seeds due to pathogenic infections which cause
more than 80% mortality, even up to 100% (Yanuhar
et al., 2012). VNN virus has been reported to infect
cultivated marine fish and has been stipulated in
Ministerial Decree number 26 Year 2013 as Pests
and Diseases of Quarantine Fish (HPIK) Group I.
VNN weakens the nervous system of fish so that the
fish will lose control nerves, will experience
weakness of motion, and eventually death (Yanuhar,
2015).
The development of local natural materials as one
of the countermeasures for controlling the spread of
the VNN virus is very much needed. Natural
Yanuhar, U., Musa, M., Arfiati, D., Caesar, N. and Junirahma, N.
In-vivo Test of Chlorella Protein Fragments as Nucleotide Vaccine Candidates in Grouper Viral Nervous Necrosis (VNN) Infection against Haematological Response.
DOI: 10.5220/0009588100790083
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 79-83
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
79

materials used are derived from natural ingredients
that have not been developed much, one of which is
the use of microalgae. Chlorella vulgaris is a type of
one-celled green microalgae that can grow and be
found in warm climates. C. vulgaris has many
ingredients in it which include protein, vitamins,
minerals, carbohydrates, fats, chlorophyll and beta
carotene (Tang dan Paolo, 2011). The use of
microalgae has been developed, especially in the
field of pharmacology. Microalgae have benefits as
antioxidants for fish because they contain vitamins,
polysaccharides, and bioactive compounds (Yanuhar,
2016).
Blood tests are conducted to establish the
diagnosis of a disease in fish because physiological
disorders in fish will cause changes in blood
components which will then be able to determine the
condition or health status of the fish (Yanuhar et al.,
2019). Based on these problems, research is needed
on the hematological analysis of groupers
(Cromileptes altivelis) infected with Viral Nervous
Necrosis (VNN) by in vivo testing using protein
fragments from C. vulgaris.
2 METHODS
This study utilized crude extracts from C. vulgaris
marine microalgae to be tested on cantang grouper
(Epinephelus sp) infected with Viral Nervous
Necrosis (VNN). C. vulgaris samples were obtained
from the Brackish Aquaculture Fisheries Center
(BPBAP) of Situbondo. The research took place at
the Laboratory of Environment and Biotechnology
Aquatic, Faculty of Fisheries and Marine Sciences,
Brawijaya University and Organic Chemistry
Laboratory, Faculty of Science and Technology,
State Islamic University of Malik Ibrahim Malang.
2.1 Extraction of C. Vulgaris
C. vulgaris was extracted by maceration using
methanol PA solvent in a ratio of 1:5 for 24 hours.
Then it was filtered using filter paper to remove the
pulp so that the extract was obtained with a solvent.
Furthermore, to obtain the extract, the solvent was
removed by using a rotary vacuum evaporator at a
temperature of 40 ºC, with a speed of 60 rpm.
2.2 In-vivo Test of C. vulgaris Extract
in Groupers
In this study, an in-vivo treatment of extracts from
C. vulgaris marine microalgae on Groupers (C.
altivelis) was carried out. The testing process was
carried out orally which refers to Yanuhar (2015),
using 5 treatments, namely (A) healthy fish, (B)
VNN-infected fish, (C) VNN-infected fish with
administration of C.vulgaris crude extracts of 17 µg
mL
-1
, (D) VNN-infected fish with administration of
C.vulgaris crude extract of 33 µg mL
-1
, and (E)
VNN-infected fish with administration of C.vulgaris
crude extract of 50 µg mL
-1
. Oral treatment with the
help of feeding tube was carried out for 3 times,
namely on day 0, 5, and 10. Each rearing tank
contained 12 groupers with a size of 10 cm and the
test treatment was carried out for 24 days.
Hemotological observations were then performed to
determine the effect of in vivo test treatments on the
Groupers.
2.3 Haematological Response
Observations of measured hematologic responses
consisted of erythrocytes, leukocytes, hemoglobin
and hematocrit. Blood samples were taken once at
the end of the study. The method of blood sampling
in fish was carried out according to Svobodova et al.
(2006). This blood sampling was carried out using a
0.5 mL syringe that has previously been added with
Ethylene Diamine Tetra Acetatic Acid (EDTA) at a
dose of 1.50 ± 0.25 mg/mL of blood. The fish was
placed with the head on the left side. Blood samples
were taken using a syringe that pierced the muscles
in the midline of the body behind the anal fin.
2.3.1 Erythrocyte Calculation
The procedure for calculating erythrocytes count
was measured according to Blaxhall and Daisley
(1973), firstly, blood was sucked with a pipette
containing a red stirrer grains to scale 1 (a pipette to
measure red blood cells count), then hayem’s
solution was added to scale 11. The stirring of the
blood in a pipette was done by swinging a hand
holding a pipette like forming a number, specifically
number 8, for 3-5 minutes so that the blood was
mixed evenly. The first two drops of the blood
solution in a pipette were removed, then the drops
were placed on a Neubauer haemocytometer and
were covered with a glass cover. Then red blood
cells count was calculated with the help of a
microscope with 400x magnification. Red blood
cells (erythrocytes) count can be calculated by the
following formula. According to Blaxhall and
Daisley (1973):
Σerythrocytesfoundx10
4
cells/mm
3
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
80

2.3.2 Leukocyte Calculation
The procedure for calculating erythocyte count was
measured according to Blaxhall and Daisley (1973),
blood samples were sucked with a pipette containing
white stirrer grains to a scale of 0.5. Then, the truk’s
solution was added to scale 11. The stirring of the
blood in a pipette was done by swinging a hand
holding a pipette like forming a number, specifically
number 8, for 3-5 minutes so that the blood was
mixed evenly (the same as stirring for the
calculation of red blood cells count). After that, the
first two drops of blood solution from the pipette
were removed, then the solution was dropped to the
haemocytometer, after which it was closed with a
glass cover. The white blood cells (leukocytes)
count can be calculated by the following formula.
According to Blaxhall and Daisley (1973):
Σleukocytesfoundx50cells/mm
3
2.3.3 Hemoglobin Calculation
Measurement of hemoglobin levels was done by
sampling the blood with a sahli pipette up to a scale
of 20 mm
3
or on a scale of 0.2 ml. Then the tip of
the pipette was cleaned with tissue paper. The blood
in the pipette was transferred into a Hb-meter filled
with 0.1 N HCl to a scale of 10 (red). The blood was
then stirred with a stirring rod for 3-5 minutes. The
distilled water was added to the tube until the color
of the blood was like the color of the standard
solution present in the Hb-meter. The hemoglobin
scale can be seen on the gr % (yellow) pathway
scale, which meant the amount of hemoglobin in
grams per 100 ml of blood.
2.3.4 Hematocrit Calculation
The examination of hematocrit values was
performed using the microhematocrit method.
Microhydematocrit with heparin was inserted into
the collected blood sample, until the blood filled
approximately three quarters (3/4) of the capillary
tube. In addition, one end of the capillary tube was
blocked by sticking it in the wax stopper. Then it
was centrifuged for 5 minutes using a
microhematocrit centrifuge with a speed of 1,500
rpm. In addition, the results were read using a
hematocrit reader and were expressed in % (Vonti,
2008).
3 RESULTS AND DISCUSSION
3.1 Erythrocyte Calculation
Based on the calculation, red blood cells count
(Figure 1.) in treatment (A) was 203 x 104
cells/mm
3
, in treatment (B) was 48 x 104 cells/mm
3
,
in treatment was 97 x 104 cells/mm
3
, in treatment
(D) was 107 x 104 cells/mm
3
and in treatment (E)
was 94 x 104 cells/mm
3
. The highest erythrocytes
count was found in treatment (A), i.e. healthy
grouper without treatment and the lowest was found
in treatment (B) VNN-infected fish.
250
203
1
0
200
cells/
mm3
150
97
107
94
100
48
Cou
nt
50
0
A
B
C
D
E
Treatment
Figure 1. Erythrocyte calculation results. (A) healthy fish,
VNN-infected fish, (C) VNN-infected fish with
administration of C.vulgaris crude extracts of 17 µg mL-1,
VNN-infected fish with administration of C.vulgaris crude
extract of 33 µg mL-1, and (E) VNN-infected fish with
administration of C.vulgaris crude extract of 50 µg mL-1.
Among the three treatments of C. vulgaris
extract tested, treatment (D) at a dose of 33 µg mL
-1
gave the highest increase in the erythrocytes count at
107 x 104 cells/mm
3
. It showed that administration
of C. vulgaris extract at a dose of 33 μg mL
-1
increased the erythrocytes count of groupers infected
with VNN. The fish blood cells count in teleostei
fish ranged from 1.05 × 106 cells/mm
3
- 3.0 x 106
cells/mm
3
. Low erythrocytes count are an indicator
of anemia, while high erythrocytes count indicates
that fish are under stress (Sababalat, 2015).
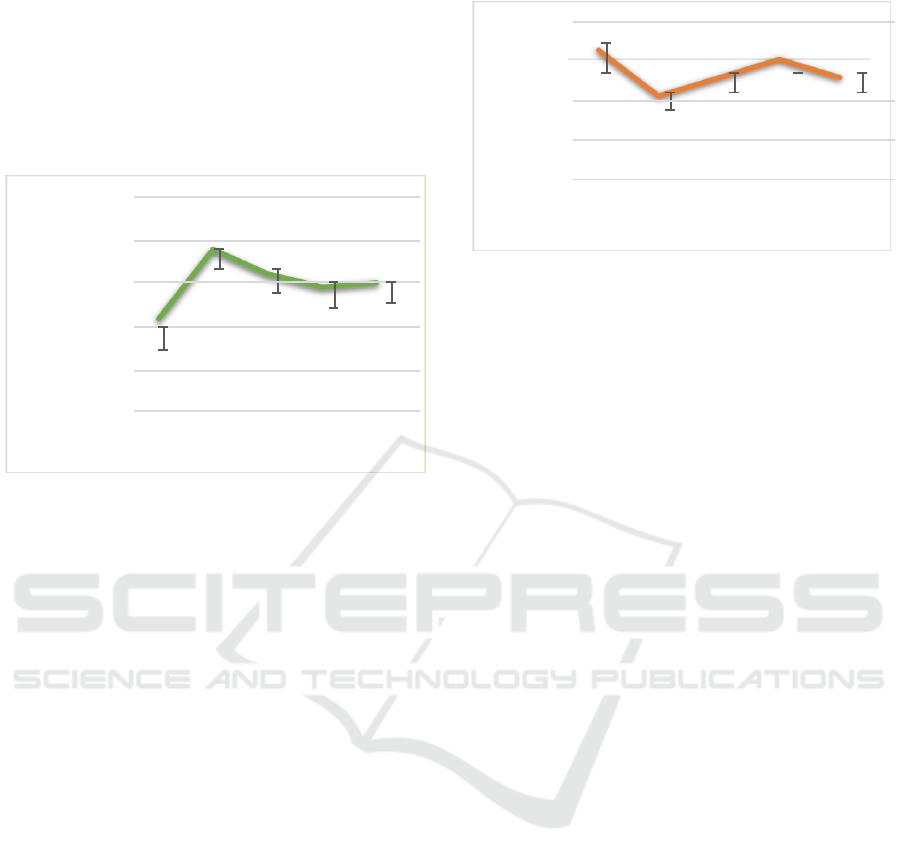
3.2 Leukocyte Calculation
White blood cells are immune cells that will respond
to the presence of pathogens or foreign objects that
enter the body, the higher the pathogenicity, the
body will produce more white blood cells. In
addition, according to Muiswinkel and Vervoorn
(2006), leukocytes have a variety of functions,
In-vivo Test of Chlorella Protein Fragments as Nucleotide Vaccine Candidates in Grouper Viral Nervous Necrosis (VNN) Infection against
Haematological Response
81
closely related to the removal of foreign matter
(including pathogenic microorganisms). Based on
the calculation of the leukocytes count (Figure 2.) it
shown the results of leukocyte calculation, as
follows: treatment (A) was 91,350 cells/mm
3
,
treatment (B) was 182,050 cells/mm
3
, treatment (C)
was 150,000 cells/mm
3
, treatment (D) was 133,300
cells/mm
3
and treatment (E) of 139,000 cells/mm
3
.
3
250,000
133,300
139,000
cells/mm
200,000
182,050
150,000
Leukocytes
150,000
50,000
91,350
O
f
100,000
A
B
C
D
E
Count
0
Treatment
Figure 2. Leukocyte calculation results. (A) healthy fish,
VNN-infected fish, (C) VNN-infected fish with
administration of C.vulgaris crude extracts of 17 µg mL-1,
VNN-infected fish with administration of C.vulgaris crude
extract of 33 µg mL-1, and (E) VNN-infected fish with
administration of C.vulgaris crude extract of 50 µg mL-1.
Leukocytes or white blood cells are an important
part of the body’s defense system which has the
nature to prey on pathogens that enter the body.
Therefore, leukocytes are very closely related to the
immune system. Leukocytes have a role in cellular
defense and humoral organisms against foreign
substances. Fish have white blood cells called
leukocytes which range from 20,000 - 150,000
cells/mm
3
(Irianto, 2005). Nearly all treatments
showed leukocyte counts in the normal category
except for VNN-infected fish.
3.3 Hemoglobin Calculation
According to Almanda et al. 2007, low Hb levels
caused the metabolic rate to decrease and the energy
produced to be low. This makes the fish weak and
has no appetite and looks still at the bottom or hangs
under the surface of the water. Normal hemoglobin
levels in fish range from 5.05 to 8.33 grams/100 ml
of blood or grams/%.
8
6.5
6
0
5 5
6
4
4
2
A
B C D
E
Treatment
Figure 3. Hemoglobin calculation results. (A) healthy fish,
VNN-infected fish, (C) VNN-infected fish with
administration of C.vulgaris crude extracts of 17 µg mL-1,
VNN-infected fish with administration of C.vulgaris crude
extract of 33 µg mL-1, and (E) VNN-infected fish with
administration of C.vulgaris crude extract of 50 µg mL-1.
The observation results of the highest hemogloblin
levels (Figure 3.) other than healthy fish treated (A)
were found in treated groupers (D) VNN-infected
fish with C.vulgaris crude extract of 33 µg mL-
1with hemoglobin levels of 6.5 gr/100 ml. The
lowest hemoglobin concentration was observed in
treatment of VNN-infected fish with a hemoglobin
level of 4 gr/100 ml.
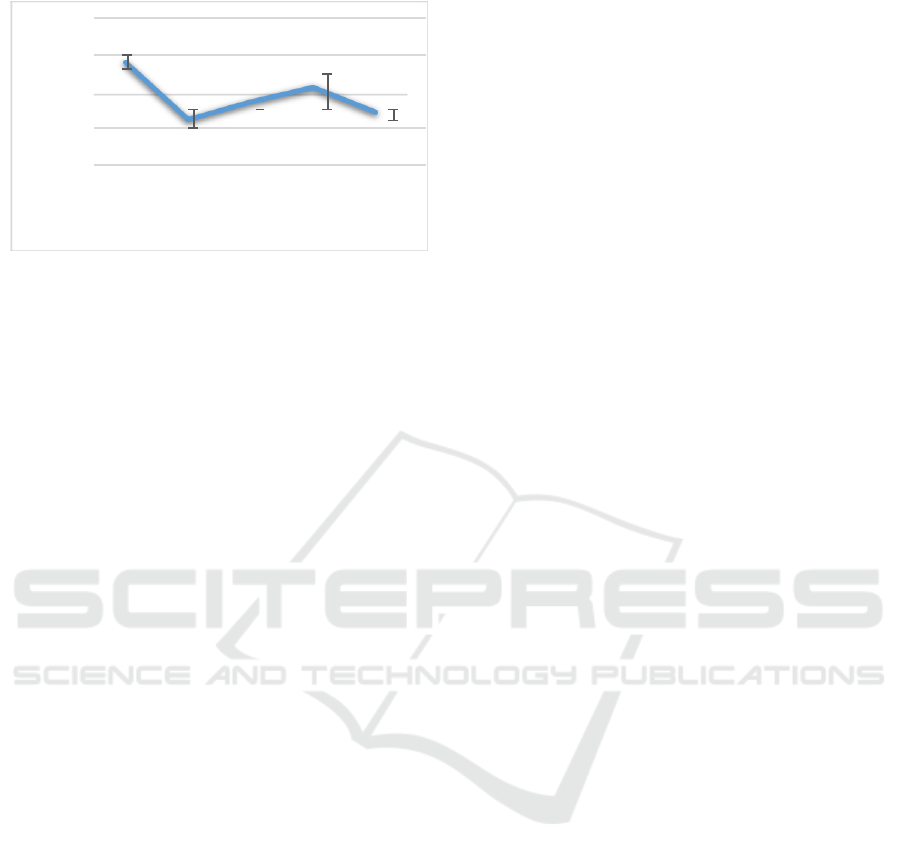
3.4 Hematocrit Calculation
Hematocrit is a comparison between red blood cells
and blood plasma, and it affects the regulation of red
blood cells. Hematocrit is a means for aquaculture to
find out whether the fish being cultivated has anemia
or not. Hematocrit is the percentage of the volume of
erythrocytes in the blood and its value is related to
red blood cells count. The increase in hematocrit
levels is influenced by two factors, namely changes
in environmental parameters, especially the
temperature and physiological conditions of fish
related to the energy needed (Jawad et al., 2004).
Based on hematocrit observations (Figure 4.) it
obtained results as follow: namely at treatment (A)
by 29%, treatment (B) by 13%, treatment (C) by
18%, treatment (D) by 22% and treatment (E) by
15% . The highest hematocrit value was in the
treated groupers (D), namely the VNN-infected fish
with C.vulgaris crude extract of 33 µg mL-1,which
was around 30%. Whereas the lowest hematocrit
value was in treatment (B), namely VNN-infected
fish, which was around 13%.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
82
(%)
40
29
Hemat
ocrit
30
18
22
10
O
f
20
13
15
Count
0
A
B
C
D
E
Treatment
Figure 4. Hematocrit calculation results. (A) healthy fish,
VNN-infected fish, (C) VNN-infected fish with
administration of C.vulgaris crude extracts of 17 µg mL-1,
VNN-infected fish with administration of C.vulgaris crude
extract of 33 µg mL-1, and (E) VNN-infected fish with
administration of C.vulgaris crude extract of 50 µg mL-1.
4 CONCLUSIONS
The health condition of fish can be seen from the
hematological status which changes the amount
value at the normal range. Based on this study, the
hematological status of groupers infected with VNN
was not good. However, based on the results of in
vivo tests conducted, it was showed that the
administration of C. vulgaris extract gave positive
results on improving the hematological status of
grouper (C. altivelis) infected with VNN with the
most optimal dose of 33 μg mL
-1
.
ACKNOWLEDGEMENTS
We offer our gratitude to the Ministry of Research,
Technology and Higher Education of Republic of
Indonesia for funding this research through the
Applied Research Excellence for University
(PTUPT) Scheme, 2019.
REFERENCES
Alamanda, I. E., N. S. Handajani, A. Budiharjo. 2007. Use
of Hematology Methods and Observation of Blood
Endoparasites for Health Determination of Dumbo
Catfish (Clarias gariepinus) in Aquaculture Pool in
Mangkubumen Village, Boyolali. Biodiversitas. 8 (1):
34–38. (In Indonesian)
Blaxhall, P.C. and Daisley, K.W., 1973. Routine
haematological methods for use with fish blood.
Journal of Fish Biology, 5(6), pp.771-781.
Irianto, A. 2005. Fish Pathology Teleostei. Gadjah Mada
University Press, Yogyakarta. (In Indonesian)
Jawad, L.A., M.A. Al-Mukhtar and H.K. Ahmed. 2004.
The Relationship between Haematocrit and Some
Biological Parameters of the Indian Shad, Tenualosa
ilisha (Family Clupeidae). Animal Biodiversity and
Conservation, 27(2):47-52.
Noor, N. Md., Simon K.D, Zaidi C.C. Mazlan A.G. 2018.
Effects of Salinities and Diets on Growth of Juvenile
Hybrid Grouper, Epinephelus fuscoguttatus × E.
lanceolattus. Turkish Journal of Fisheries and Aquatic
Sciences 18: 1045-1051.
Sudaryatma, P.E., A.T. Lestari, N.L. Sunarsih, K.S.
Widiarti, S.N. Hadayat, D. Srinoto. 2012.
Immunocytochemical Streptavidin Biotin: Early
Detection of Viral Viral Nervous Necrosis in Mucus
of Grouper Tiger (Epinephelus fuscoguttatus). Journal
of Veterinary Science. 30 (1). (In Indonesian)
Svobodová, Z., Vykusová, B., Modrá, H., Jarkovský, J.
and Smutná, M., 2006. Haematological and
biochemical profile of harvestsize carp during
harvest and post harvest storage. Aquaculture
Research, 37(10), pp.959-965.
Tang G. dan Paolo M.S. 2011. Vitamin A, Nutrition, and
Health Values of Algae: Spirulina, Chlorella, and
Dunaliella. Journal of Pharmacy and Nutrition
Sciences, 1, 111-118.
Van Muiswinkel WB, Vervoorn-Van Der Wal B. 2006.
The immune system of fish. In: PTK Woo (ed) Fish
diseases and disorders. CAB International,
Wallingford, p 678– 701.
Vonti, O. 2008. Overview of Carp Fish (Cyprinus carpio
Linn) The signal strain originating from the Ciampea-
Bogor area. Faculty of Veterinary Medicine. Bogor
Agricultural University (In Indonesian).
Yanuhar U., Gusman E., dan Arfiati D. 2012. The
Exposure Immunogenic Protein of Viral Nervous
Necrotic on Humpback Grouper That Influences to
Proliferation and Expression of Immune Cells
(Interferon γ and NFKb Cell). Advances in
Environmental Biology, 6(1): 388-396.
Yanuhar, U. 2015. Effects of Pigment-Protein Fraction
from Nannocloropsis Oculata on TNFα and IL-6
Which Act as an Anti-Inflammatory Against Viral
Nervous Necrosis (VNN) Infection. Procedia
Chemistry 14. Elsevier Ltd.: 437–43.
Yanuhar, U., 2016. Marine Microalgae: Nannochloropsis
oculata. Universitas Brawijaya Press. (In Indonesian).
Yanuhar, U., Al-Hamidy, I. and Caesar, N.R., 2019,
February. Treatment of Chlorella sp. extract on heat
shock cluster (HSC) response from the tissue and
bloodcells proliferation of Epinephelus fuscoguttatus-
lanceolatus infected by Viral Nervous Necrosis. In
IOP Conference Series: Earth and Environmental
Science (Vol. 236, No. 1, p. 012100). IOP Publishing.
In-vivo Test of Chlorella Protein Fragments as Nucleotide Vaccine Candidates in Grouper Viral Nervous Necrosis (VNN) Infection against
Haematological Response
83
