
Storage Duration Effect of Kelor Leaf (Moringa oleifera) Extracts
with Methanol against Growth of Streptococcus agalactiae and
Escherichia coli Caused Mastitis in Dairy Cattle
Puguh Surjowardojo
1
, Rachmad Dharmawan
2
, Rifai
2
and Ike Ambarwati
2
1
Lecturer at Animal Science Faculty, Brawijaya University
2
Student at Animal Science Faculty, Brawijaya University
Keywords: Antimicrobial, Mastitis, Storage, Inhibiting Capability
Abstract: The research aimed to determine the storage duration effectiveness of Moringaoleiferaleaf extract to inhibit
the growth of Streptococcus agalactiaeand Escherichia coli causemastitis on dairy cows. The materials
were Streptococcus agalactiae and Escherichia coli from Bacteriology Laboratory Agriculture Faculty,
Brawijaya University counted as 108 CFU/ml and Moringa oleifera leaf. This research method was an
experiment Completely Randomized Design 5 treatments and 5 replications. Storage duration treatment was
P0 (control), P1 (2nd day), P2 (4th day), P3 (6th day), P4 (8th day) on the same concentrations 70%. The
variable measured was the diameter of the inhibition zone. The data analyzed using ANOVA followed by
the Duncan test. The results showed that Moringa oleifera leaf extract had difference highly significant
capability to inhibit the growth of Streptococcus agalactiae and Escherichia coli (P < 0.01). The Capability
of Moringa leaf extract to maintain bacterial growth inhibition until day 2 for Streptococcus agalactiae and
Escherichia coli. Maximum storage time until day 2 to maintain the effectiveness of Moringa leaf extract in
inhibiting the growth of Streptococcus agalactiae and Escherichia coli.
1 INTRODUCTION
Mastitis is an inflammation of the udder gland in
dairy cows. Mastitis is caused by injury to the nipple
or udder tissue so that it is infected by
microorganisms (Surjowardojo, et al. 2016), mastitis
can also be transmitted to other livestock.
Surjowardojo (2011) mastitis can reduce milk
production by 4.4 - 8.3 lt /day or 28.4% - 53.5% of
total production. The decrease in production is
directly proportional to the level of mastitis, so the
higher the rate of mastitis the greater the decrease in
milk production.Manifestations of mastitis infection
can be divided into two types namely clinical and
sub-clinical. Supar and Ariayanti (2008) subclinical
mastitis caused by pathogenic microorganisms
including Staphylococcus aureus, Streptococcus
agalactiae (Tuaskal, etal. 2012), Escherichia coli and
Corynebacterium bovis. Hameed and Korwin-
Kossakowska (2006) mastitis bacteria are dominated
by Staphylococcus aureus, Streptococcus
dysagalactiae, Streptococcus agalactiae and
Streptococcus uberisand Coliform bacteria
especially Escherichia coli (Supar and Ariayanti,
2008) and Klebsiella. It has an impact on reducing
production in large numbers while the treatment of
these infections is difficult to carry out to
completion and requires a large cost in its
operations.Surjowardojo, etc.(2016) teat dipping is a
method of preventing mastitis infection by dipping
the nipple in antiseptic post milking.Moringa
(Moringa oleifera) is a native plant of Indonesia that
can be used as medicine. Moringa oleifera leaves are
a natural ingredient of antibiotics because they have
active compounds, including flavonoids (Widjawati,
et al.) saponins, tannins, alkaloids, and terpenoids
(Yudistira, et al. 2013). Moyo (2012) Moringa
oleifera leaves have antimicrobial activity against
some Gram-negative bacteria including Escherichia
coli. Moringa oleifera leaves contain secondary
metabolites such as essential oils, polyphenols, and
saponins which have potential as antibacterial and
antifungal. Fuglie (2001) added that the saponin
content is 5%, tannins are 1.4% and triterpenoids are
5%. Tannins, polyphenols, and saponins have been
known to damage bacterial cells by inhibiting
152
Surjowardojo, P., Dharmawan, R., Rifai, . and Ambarwati, I.
Storage Duration Effect of Kelor Leaf (Moringa oleifera) Extracts with Methanol against Growth of Streptococcus agalactiae and Escherichia coli Caused Mastitis in Dairy Cattle.
DOI: 10.5220/0009587901520156
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 152-156
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

protein synthesis and damaging cell
membranes.Moringa oleifera leaf extract has
potential as an antimicrobial the bacteria
Streptococcus agalactiae and Escherichia coli, it is
necessary to investigate the effect of the storage time
of Moringa oleifera leaves extract with methanol as
a solvent.on the growth of Streptococcus agalaciae
and Escherichia coli bacteria causing mastitis in
dairy cows.2 Manuscript Preparation
We strongly encourage authors to use this document
for the preparation of the camera-ready. Please
follow the instructions closely in order to make the
volume look as uniform as possible (Moore and
Lopes, 1999).
2 MATERIAL AND METHODS
2.1 Material
The materials were Streptococcus agalactiae and
Escherichia coli which were produced by the
Bacteriology Laboratory majoring in Plant Pests and
Diseases, Faculty of Agriculture, Brawijaya
University. The instruments were Moring aoleifera
leaf extract, analytical scales (acc.0.1 mg), oven,
grinder, 1-liter Erlenmeyer, measuring cup, rotary
evaporator, funnel Buchner, vacuum pump, shaker
incubator, filter paper. The instruments of bacterial
inhibition tests were Petri dishes, test tubes, Spertus
/ Bunsen lamps, autoclaves, incubators, Erlenmeyer
flasks, 500 mL measuring cups, micropipettes,
tweezers, calipers, stirrers, magnetic stirrers, label
paper, tissue, plastic wrap, L glass sticks, aluminum
foil, Cork borer. Moringa oleifera leaf extract with
96% methanol solvent Moringa oleifera leaves are
obtained from Mr. Juma'il's garden in Panarukan,
Kepanjen, Malang. Mac Conkey Media Agar,
MRSA media, 96% p.a methanol, 70% alcohol, and
Moringa oleifera leave powder.
2.2 Method
This research method was completely randomized
design (CRD) with 5 treatments and 5 replications.
The treatment used is the storage time of
Moringaoleifera leaves methanol extract at a
concentration of 70%. With the following
conditions:P0 = Storage day 0, P1= Storage day 2,
P2= Storage day 4, P3= Storage day 6, P4= Storage
day 8.
2.3 Procedure
1. Making Simplisia Moringa oleifera Leaves
2. Moringa oleifera leaves Extraction
3. Making 70% Moringa oleifera leaves Extract
Solution Concentration according to Manu (2013).
4. Making Mac Conkay Agar (MCA) Media
anonymously (2011).
5. Making Media de Mann Rogosa Sharpe Agar
(MRSA) according to Anonymously (2011).
6. Inhibitory testing accordingly (Kasogi et al,
2014):
Warbung, et al (2014) the formula for calculating the
inhibition zone is as follows:
Note:
d1 = vertical diameter of the clear zone on the
media.
d2 = horizontal diameter of the clear zone on the
media.X = well hole (5 mm).
Susanto, etal. (2012) inhibitory zones can be
categorized as follows, for diameters> 20 mm are
categorized as highly strong, 11-20 mm are
categorized as strong, 6-10 mm are categorized as
moderate and <5 mm are categorized as weak.
2.4 Variable
The variables were diameter of inhibitory zone in
the form of clear area on the surface of the medium
between the extract of Moringaoleifera leaves and
Streptococcus agalactiae and Escherichia
colibacteria.
2.5 Data Analysis
The research method used was a completely
randomized design experimental method with 5
treatments and 5 replications. The results of the data
obtained were processed using ANOVA followed by
Duncan's Multiple Range Test is performed.
Storage Duration Effect of Kelor Leaf (Moringa oleifera) Extracts with Methanol against Growth of Streptococcus agalactiae and
Escherichia coli Caused Mastitis in Dairy Cattle
153
3 RESULTS AND DISCUSSION
3.1 Inhibition Zone of Streptococcus
agalactiae Bacterial
The results of the research of the effect of the
storage duration of Moringa oleifera leaves
methanol extract on the bacteria Streptococcus
agalactiae are shown in Table 1.
Table 1: Inhibitory zone diameter in Streptococcus
agalactiae bacteria.
Treatment Inhibition Zone Categories
T0 (Control) 22.53 ± 0.42
c
Highly strong
T1 (Days-2) 22.24 ± 0.29
c
Highly strong
T2 (Days-4) 21.62 ± 0.49
b
Highly strong
T3 (Days-6) 20.76 ± 0.25
a
Highly strong
T4 (Days-8) 20.34 ± 0.58
a
Highly strong
Note: Different superscripts in the same column show
significant between treatments (P <0.01).
Extract storage of days 0 days-2 showed that the
first and second mean values were not significantly
different, and were significantly different from the
4th, 6th and 8th-day treatments. Storage of methanol
extract of Moringa oleifera leaves is recommended
until the 2nd day (P1). Al-Zubaydiet al. (2009) that
flavonoids have broad antibacterial activity because
of their complex ability to extracellular and soluble
proteins as well as to precipitate proteins on the cell
walls of the bacterium Kiarostami et al, (2010). In
addition, these phenol compounds tend to form
hydrogen bonds with cell wall proteins so that they
can destroy cell membranes in bacteria. The graph of
the reduction in diameter of the inhibition zone of
methanol extract of Moringa oleifera leaves is
shown in Figure 1.
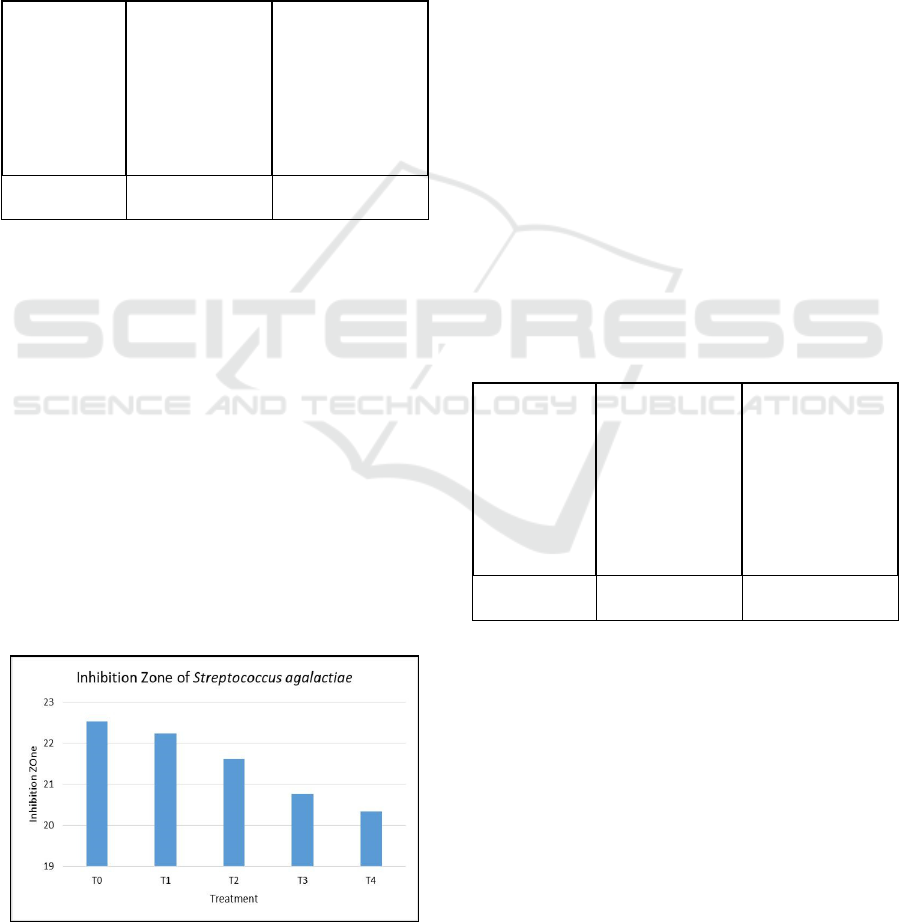
Figure 1: Inhibition zone of Streptococcus agalactiae.
Inhibition zone of Streptococcus agalactiae
bacteria during storage has decreased, especially
temperature factors. If the extract is stored at room
temperature, the extract will quickly evaporate and
cause the effectiveness of the extract to decrease
bacterial growth. This is in accordance with Siswadi
(2002) Decreasing the effectiveness of antimicrobial
compounds is influenced by many factors including
the type, age and state of microbes, concentration of
antimicrobial substances, temperature and contact
time, as well as the physicochemical properties of
the substrate such as pH, water content and surface
tension, number of components existing and other
factors. Klimczak (2006) andSuhartatiket al. (2012)
that the higher the storage temperature, the lower the
flavonoid and phenolic content of the extract. The
storage of plant extracts affects uterine activity and
depends on extraction temperature and storage
temperature.
3.2 Inhibition Zone of Escherichia coli
Bacterial
The results of the study of the effect of the storage
duration of Moringa oleifera leaves methanol extract
on Escherichia coli are shown in Table 2.
Table 2:Inhibitory zone diameter in Escherichia coli
bacteria.
Treatment Inhibition Zone Categories
T0 (Control) 22.04 ± 0.59d
Highly strong
T1 (Days-2) 21.50 ± .49cd
Highly strong
T2 (Days-4)
20.59 ±
0.74bc
Highly strong
T3 (Days-6) 19.59 ± 0.64b
Strong
T4 (Days-8) 18.13 ± 0.46a Strong
Note: Different superscripts in the same column show
significant between treatments (P <0.01).
Extract storage of days 0 days-2 shows the first
and second mean values were not significantly
different and were significantly different from the
treatment on days 4, 6 and 8. Storage of methanol
extract of Moringa oleifera leaves is recommended
until the 2nd day (P1). The inhibitory ability of
Moringa oleifera leaves methanol extract against
Escherichia coli is weaker than that of Streptococcus
agalactiae. The capability of methanol extract of
Moringa oleiferaleaves at a concentration of 70% is
quite strong. Given the cell wall of gram-
negativebacteria is more complex than the structure
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
154
of gram-positive bacteria. This is consistent with the
explanation Suwito (2010), that gram-negative
bacteria have a cell wall that consists of three layers.
With a more complex structure of gram-negative
bacteria according to Harijani (2009), making
antibiotic compounds more difficult to enter the cell.
In addition to the effect of shelf life, the decrease in
inhibition is also due to other factors such as type,
age, antimicrobial concentration, microbial state,
and physicochemical properties such as pH, water
content (Siswadi, 2002). A graph of the decrease in
the inhibition zone diameter of methanol extract of
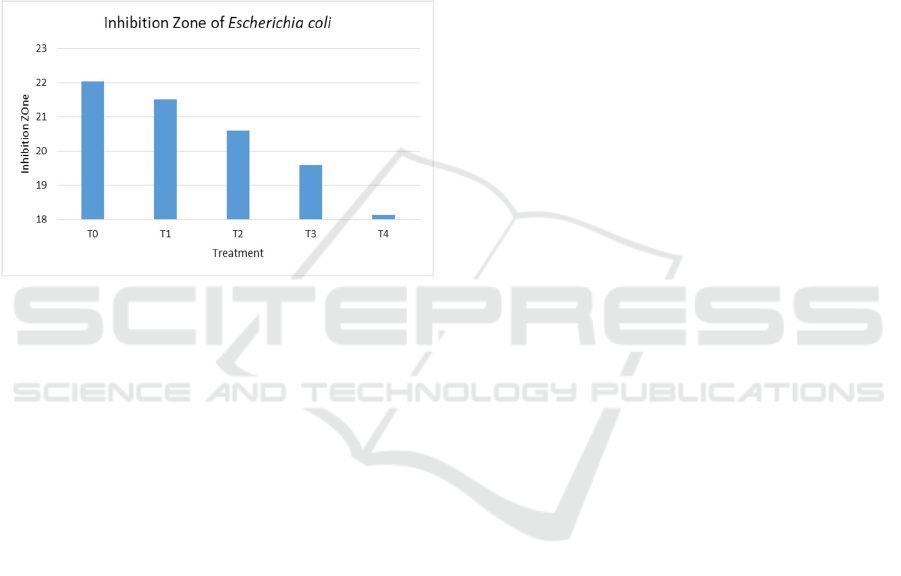
Moringa oleiferaleaves is shown in Figure 2.
Figure 2: Inhibition zone of Eschrichia coli.
Compounds contained in Moringa leaf extracts
such as flavonoids, saponins, and tannins have a
function in damaging cell walls in bacteria. This is
in accordance with the opinion of Khasanah, etc.
(2014) Cell walls are the main target of being
attacked by antibacterial substances contained in the
Methanol extract of Moringa leaves, making it easier
for tannins, saponins and flavonoids to enter the cell
membrane. Cell walls are not selectively permeable
so that these compounds are easily penetrated
through the cell wall which will cause disruption of
the integrity of the bacterial cell wall. The ability of
flavonoids as an antibacterial is able to stick to
bacterial cell walls and disrupt bacterial membranes,
so bacteria become lysis and die. The ability of
flavonoids to provide antibacterial effects includes
inhibiting the function of cytoplasmic membranes,
inhibiting nucleic acid synthesis, and inhibiting
antibacterial activity by inhibiting energy
metabolism, flavonoids inhibit oxygen consumption
by interfering with the electron transport chain
respiration. Permatasari, et al (2013) added that
Saponin is included in the antibacterial group which
interferes with the permeability of microbial cell
membranes. Causing damage to cell membranes and
causing the release of various important components
from the microbial cells, namely proteins, nucleic
acids, nucleotides, and others.
REFERENCES
Al-Zubaydi, Sami R., Al-Hmdany, Maetham A., And
Shyma'a J. Raesan. 2009. Antibacterial Effect Of
Some Medicinal Plant Extracts Against Some
Pathogenic Bacterial Strains. The 2nd Kurdistan
Conference on Biological Science.
Anonymous.2011.MicrobiologyLaboratoryEquipment.//al
atalatlaboratorium.com/LaboratoriumMikrobiologi/ma
c-conkey-agar.November 1, 2019.esJ.Duhok Univ.:
12, 244-249.
Fuglie, L. J. 2001. The Miracle Tree (The Multiple
Attributes of Moringa). Senegal: CWS Dakkar.
Hameed, S. and Korwin-Kossakowska. 2006. Public
Health Hazard Due To Mastitis In Dairy Cows.
Animal Science Pap Reports. 25(2):65-74.
Harijani N., M. Selomashar and B.C Tehupuring,. 2009.
The Effects of Bacteriocin Giving by
Pediococcuspentosaceus Lactic Acid Bacteria as
Nipples for Sub-Clinical Mastitis. Vol. 2(2): 257-268
Kasogi, I., Sarwiyono, and P Surjowardojo. 2013.
Methanol extract of cherry leaves (Muntingiacalabura
L) as a natural antimicrobial against Staphylococcus
aureus bacteria in dairy cows in the ngantang area,
Malang. Brawijaya University Malang
Khasanah, I., Sarwiyono and P. Suryowardojo. 2014.
Ethanol Extract of Kersen Leaves (Muntingiacalabura
L.) As Antibacterial Against Streptococcus Agalactiae
Causes Subclinical Mastitis in Dairy Cattle.Tropical
Animal Production. 15(2): 7-14.
Kiarostami, Kh.,Mohseni, R., Saboora, A. 2010.
Biochemical Changes of Rosmarinus Officinalis
Under Salt Stress. Journal ofStress Physiology &
Biochemistry.6: 114 122.
Klimczak, I., Maecka, M., Szlachta, M., Gliszczyn, A.
2006. Effect of Storage on The Content of
Polyphenols, Vitamin C and TheAntioxidant Activity
of Orange Juices. Elsevier. 20(4): 313-322.
Manu. 2013. Antibacterial Activity of Beluntas
(Plucheaindica L) Ethanol Extract Against
Staphylococcus aureus, bacillus subtilis and
pseudomonas aeruginosa. Surabaya university
scientific journal 2 (1): 1-10
Moyo 2012. Antimicrobial activities of Moringaoleifera
Lam leaf extracts. African Journal of Biotechnology
11 (11): 2797-2802.
Permatasari, G. A. A. A., Besung I. N. K. danMahatmi H.
2013. Inhibition Power of Soursop LeavesAgainst the
growth of Escherichia coli bacteria. Indonesia
MedicusVetreinus. 2(2): 162-169.
Siswadi, I. 2002. Study of Antimicrobial Activity of
Andaliman Fruit Extract (Zanthoxilumacanthopodium
D.C) Against Microbial Pathogens and Food
Destruction. [Thesis].Faculty of Agricultural
Technology. Bogor Agricultural Institute.
Storage Duration Effect of Kelor Leaf (Moringa oleifera) Extracts with Methanol against Growth of Streptococcus agalactiae and
Escherichia coli Caused Mastitis in Dairy Cattle
155

Suhartatik, N., Karyantina, M., Mustofa, A., Cahyanto, M.
N., Raharjo, S., and Rahayu, E. S. 2013. Stability of
Anthocyanin Extract of Glutinous Rice (Oryzasativa
var. Glutinosa) Black During the Process of Heating
and Storage. Agritech Journal, Vol. 33 (4): 384-390.
Supar and T. Ariyanti. 2008. Subclinical Mastitis Control
Study in Dairy Cattle. Indonesian Center for
Veterinary Research, Bogor.
Surjowardojo, P. 2011. Rate of Occurrence of Mastitis
with Whiteside Test and FriesienHolstein Dairy Cow
Milk Production.Tropical Animal Production. 12(1):
46-55
Surjowardojo, P. T. E. Susilorini, and V. Benarivo. 2016.
The Impact Of ManalagiApel Skin (Malus Sylvestris
Mill) On The Growth Of Escherichia Coli And
Streptococcus Agalactiae Causing Mastitical Causes
In Dairy Cow. 17(1): 11-21.
Susanto, D., Sudrajat and Ruga, R. 2012. Study of Active
Ingredients of MerantiMerah (ShorealeprosulaMiq) as
a Source of Antibacterial Compounds. 11 (2): 1-5.
Suwito and Widodo. 2010. Monitoring of Salmonella Sp
and Escherichia Coli in Animal Feed Materials
Salmonella sp and Escherichia coli monitoring in the
animal feed ingredients. Farm bulletin. 34 (3): 165-
168.
Tuaskal, B. J., S. Estuningsih, F. H., Pasaribu and I. W. T.
Wibawan. 2012. Orientation Dose of Streptococcus
agalactiae Irradiation for Sub Clinical Mastitis
Vaccine Material in Dairy Cattle. Scientific Journal of
Isotope and Radiation Applications 8 (2): 83-88
Warbung, Y. Y., Vonny, N. S. W., dan Jimmy. 2014.
Inhibition of Sea Sponge Extract. In Dairy
Cows.Repository.Faculty of Animal Husbandry
University of Brawijaya. Malang.
Yudistira, F. A., S. Murwani and P. Trisunuwati. 2013.
Antimicrobial Potential of Moringaoeifera Leaf Water
Extract Against Salmonella Enteritidis (SP-1-PKH) In
Vitro. Veterinary Medicine Program, University of
Brawijaya. Malang.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
156
