
Effect of Therapy on Mangosteen (Garcinia Mangostana L.) Bark
Extract on Serum Blood Blood Protease Activity and Expression of
Malondialdehyde (MDA) on Rattus Norvegicus Traumatic Brain
Injury Model
N. Indah Ratnasari
1*
, A. Aulanni’am
2
, and C. Mahdi
3
1
Institute Biosains, Brawijaya University
2
Faculty of Veterinary Medicine, Brawijaya University
3
Chemistry Department, Faculty of Mathematic and Natural Science, Brawijaya University
Keywords: Garcinia mangostana L., malondialdehyde (MDA), protease activity, inflammation, oxidative stress,
traumatic brain injury
Abstract: Traumatic brain injury is a cause of impaired cognitive and physical function that is permanent or temporary
and is accompanied by loss or change of level of consciousness and cause damage or death of cells in the
brain. Cell death or apoptosis can cause inflammation and oxidative stress andproduce reactive oxygen
species (ROS). The use of mangosteen peel extract serves to prevent inflammation and inhibit the
production of oxidative stress. This study aims to determine the role of mangosteen peel extract therapy in
brain organ expression and protease activity in rat blood serum. Besides that, minocycline is used as a test
standard and comparison of mangosteen peel extract. The results of this study formed 4 treatment groups
namely the negative group, the positivegroup TBI model, the TBI group with mangosteen skin extract
therapy dose 0.5 mL/day for 5 daysand the TBI group with minocycline therapy 0.5 mL/day dose for 5 days.
The results showed that the treatment of mangosteen peel extract after brain injury could reduce the
expression ofmalondialdehyde (MDA) by 32.29% and protease activity in rat blood serum by 47.62%.
Theresults of statistical analysis of MDA exposure and protease activity showed that there were
verysignificant differences between treatment groups (p <0.01). This study can be concluded
thatmangosteen peel extract therapy can be used when a traumatic brain injury occurs.
1 INTRODUCTION
Brain injury is one of the highest causes of death in
traffic accidents. Indonesia itself has a 50% increase
in mortality rate every year. Brain injury is a major
factor causing death in traffic accidents (Warpani,
2002).
Rats (Rattus novergicus) are animals in the form
of mammals. This white rat belongs to the Muridae, the
genus Rattus with the order of Rodentia. Also, it is an
experimental animal that is widely used in research.
This is because rats have a high similarity of 95% of
the same genes as humans. This white rat also has a
short generation rate of 1-year rats equal to 30 years of
humans with high reproduction and can manipulate
genomes directly (Armitage, 2004). This then becomes
the basis of research to observe brain injuries that
occur in humans by making mice as a model of
Traumatic Brain Injury. Mangosteen extract is
known to contain active xanthones, flavonoids, etc.
Where xanthone is a substance that has important
benefits such as anti-inflammatory, antioxidant, anti-
cancer, and also anti-cardioprotective (Miryanti et
al., 2011). The anti-inflammation contained in the
mangosteen extract is very important to be used in
traumatic brain injury because it can prevent the
onset of increased inflammation. Increasing
inflammation will indicate the body's physiological
response to an interruption by external factors
(Murfu’ati et al., 2014). Besides that, the flavonoid
content in mangosteen peel extract also has an
important effect which can inhibit the work of
enzymes involved in the formation of ROS (Redha,
2010). Based on the results of research that has been
done, it is stated that flavonoids are a group of
phenolic compounds that have antioxidative
Ratnasari, N., Aulanni’am, A. and Mahdi, C.
Effect of Therapy on Mangosteen (Garcinia mangostana L.) Bark Extract on Serum Blood Blood Protease Activity and Expression of Malondialdehyde (MDA) on Rattus norvegicus Traumatic
Brain Injury Model.
DOI: 10.5220/0009587401330140
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 133-140
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
133
properties and play a role in preventing cell damage
and cellular components caused by free radicals
(Simamora, 2009).
Some things to be able to prove that mangosteen
peel extract is a therapy that can provide positive
changes for traumatic brain injury that is proven by
using levels of malondialdehyde (MDA). This
substance is widely known and is also often used as
a biological marker of lipid peroxidation and
oxidative indicators. Also, it is easily obtained in the
blood circulation and is the main product of the
reaction of free radicals with phospholipids. The
results of these reactions are produced in constant
accordance with the proportion of lipid peroxidation
that occurs, so it is a good indicator to see the speed
of lipid peroxidation in vivo. The higher levels of
MDA are expected to increasingly show the level of
damage that occurs in traumatic brain injury (Yigit
et al., 1999).
Also, observations were made on protease
activity. Protease activity itself is the ability of
protease to break down proteins. Protease is secreted
into tissues involved in the mechanism of tissue
damage. Excessive protease activity can damage
tissue cells found in the brain (Hantoko and Drajat,
2003). Increased uncontrolled protease activity
causes an inflammatory process so it is expected to
be inhibited by mangosteen peel extract therapy.
2 MATERIAL AND METHOD
2.1 Preparation of Animals Trying
Mice (Rattusnovergicus)
Rats were divided into 4 treatment groups, each group
consisted of 5 mice. Before being treated, mice were
adapted to the laboratory environment for 7 days by
standard feeding to all mice.
Group 1 was a negative control group of rats,
namely the group of
rats without TBI induction and
the administration of minocycline therapy and
mangosteen extract. Group 2 was a positive control
rat group, namely the TBI induction rat group.
Group 3 was a group of TBI induction mice and
treated with minocycline at a dose of 0.5 mL/day.
Group 4 is a group of TBI induction mice and
mangosteen extract treated with a dose of 0.5
mL/day. The research scheme can be seen in
appendix 1.
Table 3.1: Design of the Rat Treatment Group(Rattus
norvegicus)
The research sample used was a rat animal
(Rattus norvegicus) male Wistar stain with a
bodyweight of 300-350 grams. Calculation of the
number of samples can use the Federer formula as
follows [45]:
t (n - 1) ≥ 15
4 (n – 1) ≥ 15
4n – 4 ≥ 15
4n ≥ 19
n ≥ 4, 75 (be rounded 5)
Note:
t : number of treatment groups
n : number of repetitions needed
Based on the estimation calculation from the
sample above, then for the four treatment groups
required at least 5 replications in each group so that
the total number of rat animals needed was 20.
Rats were kept by
the
treatment group and kept
in a room temperature of
22-24
o
C and humidity 50-
60% with
adequate ventilation, where each cage
consisted of 5 mice. The mouse cage is made of a
plastic tub with a size of 17.5
x 23.75 x 17.5 cm
which is equipped with a cover of
wire.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
134

The variables observed in this study are as
follows:
1. Independent variable
: treatment of falling brain
load, therapeutic
dose o
f
mangosteen extract
2. Dependent variable : brain
organ,
serum
protease
activity,
MDA
immunohistochemistry
3. Control variables : sex, age, body weight
of Rattus norvegicus
strain Wistar rats.
2.2 Providing Mangosteen and
Minocycline Skin Extract Therapy
Minocycline therapy was given to the KB TB post-
treatment group at a dose of 0,5 mL/day for 5 days.
While mangosteen therapy was given to the KC
treatment group at a dose of 0,5 mL/day for 5 days.
2.3 Preparation of Animal Rats (Rattus
novergicus) TBI Models
Ketamine at a dose of 100 mg / KgBB and xyla at a
dose of 10mg / kg BW are anaesthetized through
intramuscular injection of the thigh muscle. Then the
rats were placed face down on the surgical board and
fixed the four extremities using a paper clip. The
rat's head was disinfected using 70% alcohol and the
rat's head was shaved. The scalp of the rat is opened
with scissors from the middle to between the two
ears toward the frontal until the skull is visible. Then
the mouse head is positioned just below the cylinder
sleeve with a distance of 1 cm. The iron cylinder
weighing 40 grams and 4 mm diameter was dropped
perpendicularly from a height of 180 cm 1 time.
Then the scalp is cleaned, stitched back and given a
topical 10% gentamicin ointment and intramuscular
analgesic.
2.4 Intake of Rat Brain
Rats were treated with euthanasia using ketamine at a
dose of 0,2 mL and placed on a surgical board. Next,
cut the back of the rat's neck or cut in the direction
of the back to the abdomen entirely so that you can
see the boundary between the skull and skin.
The scalp of mice in the TBI lesion area is
completely removed. Then the mouse skull is cut as
needed from the direction of the neck intersection.
The skull was opened with the power of a finger to
open and obtained a cross-section of the brain and its
limits. Nerves that are still connected to the brain are
cut. Then the brain is carefully removed and placed
in an organ bottle containing 10% formalin Solution.
2.5 Retrieval of Rat Blood Serum
Rats were placed in a dorsal lying position on the
surgical board. Then dissected in the abdominal
cavity and taken as much as 5 cc of blood plasma in
the superior vena cava of the heart. Then put in a red
vacutainer.
2.6 Making Slides of Brain Tissue
Histopathology Preparations
The rats head was cut into 2 cm x 1 cm x 3 cm size
and brain tissue was fixed using 10% formalin, then
soaked for 18-24 hours. Then washed with running
water for 15 minutes and dehydrated using acetone
Solution for 1 hour 4 times. Then the stage of
clearing (clearing) using xylol for 30 minutes as
much as 4 times. Furthermore, the stage of
immersion (impregnation) using liquid paraffin with
a temperature of 55
o
C for 1 hour 4 times. Casting
(blocking) is done on the paraffin block and sliced
on a network that has been embedded in the paraffin
block using a rotary microtome with a thickness of
3-5 microns and placed on a glass object.
2.7 MDA Measurement by
Immunohistochemistry
Preparation slides before being deprived are heated at
60
o
C for 60 minutes. The preparations were depolished
with xylol for 2 times 10 minutes, put into absolute
ethanol for 2 times 10 minutes. Then put into
multilevel ethanol (95%, 90%, 80%, and 70% and
distilled water) for 5 minutes each. The slide
preparation was immersed in a Chamber containing
citrate buffer pH 6.0. Then the Chamber is heated in a
water temperature of 95
o
C for 20 minutes. Slides are
removed from the water bath, wait until the room
temperature ± 20 minutes. Slides were washed with
PBS for 6 minutes.
On the first day the slides were ready for IHC,
3% H
2O2 in methanol was incubated for 15 minutes
and washed with PBS pH 7.4 3 times 2 minutes. In
Unspecific Blocking Protein Background Sniper
drops, incubated 15 minutes at room temperature
then washed with PBS pH 7.4 3 times 2 minutes.
Primary antibody (MDA) drops were Solutiond in
PBS + 2% BSA buffer, incubated overnight at 4oC.
Then on the second day, the two slide preparations
were removed, waited for room temperature, then
Effect of Therapy on Mangosteen (Garcinia mangostana L.) Bark Extract on Serum Blood Blood Protease Activity and Expression of
Malondialdehyde (MDA) on Rattus norvegicus Traumatic Brain Injury Model
135

washed with PBS pH 7.4 3 times 2 minutes.
Secondary antibody is dropped, incubated 30
minutes at room temperature and washed PBS pH
7.4 3 times 2 minutes. Furthermore, SA-HRP
(StrepAvidin-Horse Radish Peroxidase) was added
and incubated for 20 minutes at room temperature.
Washed with PBS pH 7.4 3 times 2 minutes and
rinsed with distilled water. Added DAB (Chromagen
DAB: DAB buffer = 1:50) and incubated for 3-10
minutes at room temperature.
Washed with PBS pH 7.4 3 times 2 minutes and
washed with aqua dest 3 times 2 minutes.
Counterstrain (Mayer's Hematoxylin) was given
with a tap water in a ratio of 1:10 and incubated for
5-10 minutes at room temperature, then rinsed with
tap water. Mounting glass cover. Furthermore, dried
until dried. Furthermore, observations were made
using a microscope. MDA levels in rat brain tissue
can be calculated with a portrait microscope at the
Anatomy Pathology Laboratory, Faculty of
Medicine, Brawijaya University. The preparations
are placed on a microscope with 10x ocular
magnification and 10x objective magnification.
After brain tissue is seen, the objective
magnification is increased to 40x. Observations were
made on 10 different fields of view so that the
results obtained are objective.
2.8 Measurement of Protease Enzyme
Activity (serum) Protein Isolation
Blood serum is prepared first and added a little
quartz sand. After homogenate added with PBS-
Tween: PSMF (9: 1) Solution as much as 1 mL and
transferred into a sterile effendorf tube. Followed by
vortexing for 15 minutes (6000 rpm), and 10
minutes sonicated with a sonicator. Then the
supernatant is taken and added to absolute cold
ethanol in a ratio of
1 and left overnight to form a precipitate. After
that centrifuge for 15 minutes (10.000 rpm), the
sediment is taken and dried until the ethanol odour
disappears. Then the precipitate was added with a
0.02 M Tris-HCl pH 6.5 cold Solution with a
volume ratio of 1: 1.
2.9 Making the Tyrosine Raw Curve
The first step in making the tyrosine standard curve
is to prepare 10 volumetric flasks and each filled
with 20 ppm tyrosine standard Solution 1, 2, 3, 4, 5,
6, 7, 8, 9, 10 mL for concentrations of 2, 4, 6, 8, 10,
12, 14, 16, 18, 20 ppm. Next, add distilled water to
the boundary mark, then the tube is closed with
aluminium foil and shaken. Then the absorbance is
measured in each standard Solution at the maximum
wavelength. The blank used is aquades.
2.9 Measurement of Protease Activity
in Protein Isolation
The first step that must be done is to mix 500 ppm
casein as much as 200 µL, phosphate buffer Solution
pH 7 as much as 300 µL and protease enzyme as
much as 100 µL then let stand 60 minutes at 37
o
C in
the incubator. Then a 400% TCA Solution of 400%
was added and allowed to stand for 30 minutes at
27
o
C (room temperature). Then it is rotated with a
centrifuge at 4000 rpm for 10 minutes. The
supernatant was taken as much as 100 µL and
diluted 5 times the sample volume with phosphate
buffer then measured its absorbance value at a
maximum tyrosine of 280 nm. The blank used was
made by the same procedure as the determination of
activity, but for the addition of TCA treatment was
carried out as soon as possible after the addition of
the enzyme Solution. One unit of activity is as much
as tyros avoided the breakdown of 1 mL of the
protease enzyme.
Measurement of protease enzyme activity was
carried out based on the Walter (1984) method using
the formula :
Enzyme Activity
Tirosin
v
x
fp
MrTirosin
p
xq
q = incubation time
(mL) fp = dilution factor
p = amount of enzymes (mL)
2.10 Data Analysis
Analysis of quantitative immunohistochemical data
using MDA levels in rat brain and calculation of
protease activity as an anti-inflammatory marker on
brain tissue was performed statistically using a one-
way analysis of variance (ANOVA) variance test.
Then the Honestly Significant Difference (BNJ)
or Tukey test is performed to determine whether
there is a significant difference with a significance
level of 5% using Microsoft Office Excel and
statistical package for the social science (SPSS)
version 16.0 for Windows 7.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
136

3 RESULT AND DISCUSSION
In this research, therapy on traumatic brain injury
rats using mangosteen extract and minocycline as
gold standard. Observations made were the
expression of malondialdehyde (MDA) in rat brain
organs and serum protease activity in the blood.
3.1 MDA Expression of White Rat
(Rattus novergicus) Brain TBI
Model in Mangosteen Skin Extract
Therapy
Figure 1. Negative Control (Healthy)
Figure 2. TBI Positive Control
Figure 3. TBI Control and Mangosteen Skin Extract
Therapy
Figure 4. TBI Control and Minocycline Therapy
In rats, traumatic brain injury occurs aninflammatory
process and activates inflammatory mediators such
as T cells. T cells express CD4 + to recognize
antigens. T cellsactivate macrophages to produce
proinflammatory cytokines [47]. Reactive Oxygen
Species (ROS) are also produced during the
inflammatory process due to free radicals. Oxidative
stress on the central nervous system is very deadly
because the human brain mainly uses oxidative
metabolism. Although the brain weighs only 2% of
body weight, the brain uses about 50% of all body
oxygen. Another very dangerous factor is oxidative
stress in the brainwith a high PUFA
(polyunsaturated fatty acid) content, affecting almost
50% of all brain tissue structures. Poly Unsaturated
Fatty Acid is degraded by free radicals which are
ROS products such as hydroxyl radicals (-OH),
superoxide radicals (O2-), hydrogen peroxide
(H2O2) then form malondialdehyde (MDA) [48].
High MDA levels indicate that cells experience
oxidative stress [49] and are indicated by changes in
the appearance of mouse brain cells in brown colour.
Mangosteen peel extract has antioxidant and anti-
inflammatory properties. Antioxidants will inhibit
the process of activation of inflammatory cells so
that the activation of macrophages in producing
cytokines is also reduced and inhibited the
production of free radicals. Inhibition of free
radicals by antioxidants by the bioactive content of
mangosteen through the process of inhibiting the
oxidation reaction to reduce the expression of MDA.
The content of bioactive compounds possessed by
mangosteen peel extract can reduce MDA
expression better than minocycline because
minocycline is only a derivative of tetracycline so
that minocycline has only one benefit to reduce
MDA expression in TBI model mice. Minocycline is
also a drug that has long been used for the treatment
of brain injuries. This can be proven in Table 4.1.
Table 4.1 Average number of cells expressing
malondialdehyde (MDA) in TBI mouse brain organs.
Treatment group
MDA Expression
Average (%)
Negative
Control
7,87 ± 0,09
(Healthy) (A)
TBI Positive Control 12,48 ± 0,29
(B)
TBI Control and 2,16 ± 0,37
Mangosteen
Skin
Extract Therapy (C)
TBI Control and 8,45 ± 0,53
Minocycline
Therapy (D)
Effect of Therapy on Mangosteen (Garcinia mangostana L.) Bark Extract on Serum Blood Blood Protease Activity and Expression of
Malondialdehyde (MDA) on Rattus norvegicus Traumatic Brain Injury Model
137
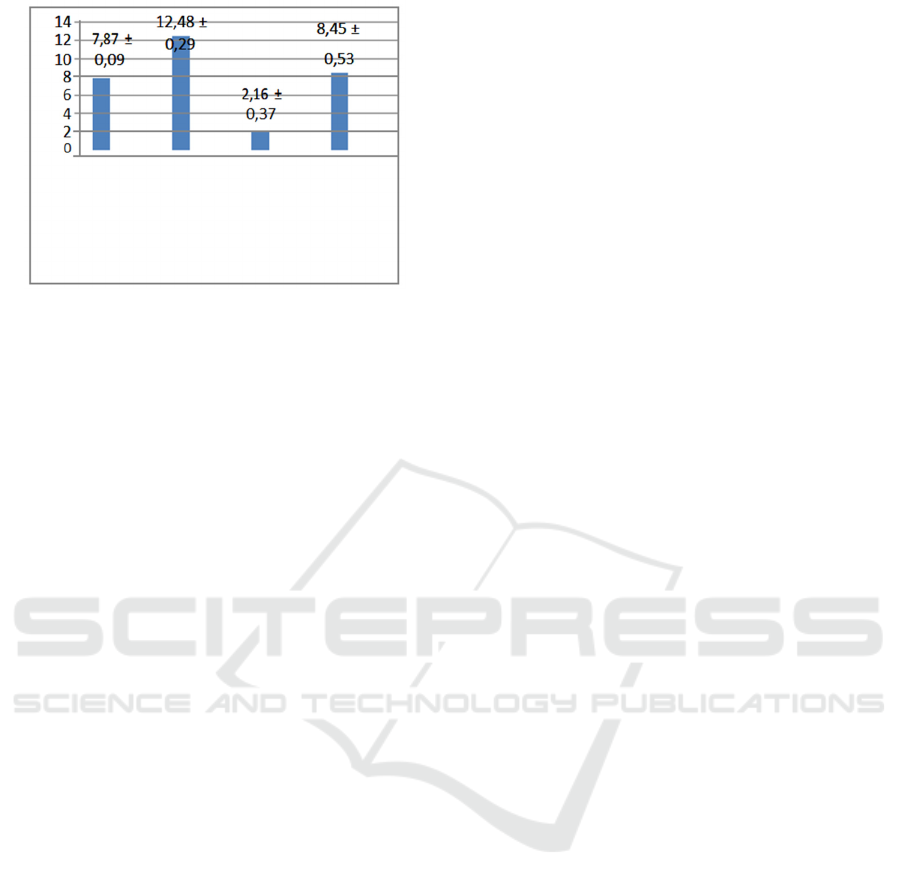
Figure 5. Graph of the number of MDA expressions in rat
brain
The average MDA level in group A was 7.87 ±
0.09. These values indicate the standard value of
MDA levels in mice under normal circumstances.
The mean value of MDA levels in group B is the
highest value of 12.48 ± 0.29 and testing by BNJ test
shows that there are significant differences
compared to treatment groups A, C, and D.
The results of statistical analysis (Appendix 11)
indicate that group B provide a real influence on the
increase in MDA expression in rat brain. The results
showed that a drop of iron cylinder weighing 40
grams and a diameter of 4 mm can cause
inflammation in the rat brain. Therapeutic groups
namely group C and group D showed a decrease in
MDA expression after administration of mangosteen
and minocycline skin extract therapy. But in the
therapy done, mangosteen skin therapy is better than
minocycline therapy. This can be caused because
there is a high antioxidant content in mangosteen
peel extract which can counteract free radicals which
is indicated by a decrease in MDA levels in the
brains of mice that have been injured. Antioxidants
in the content of mangosteen peel extract functions
as a revenger (catcher) of free radicals so that it can
help reduce high levels of free radicals in the rat
brain due to traumatic brain injury. The mechanism
of inhibition of free radicals by antioxidants
mangosteen rind extract is inhibiting the oxidation
process by inhibiting the initiation and propagation
of oxidation reactions from free radicals and ROS.
Antioxidants in mangosteen peel extract namely
xanton can contributehydrogen atoms to capture
hydroxyl radicals (OH) so as not to become reactive
so that it inhibits free radicals. The xanton
component works through the capture of O-peroxide
nitrite (ONOO-) formed from nitric oxide (NO) with
superoxide (O2-) which is a free radical. The
antioxidant content of mangosteen peel extract
inhibits the initiation process so as to prevent the
formation of lipid radicals that are unstable due to
the loss of one hydrogen atom (H) from the lipid
molecules due to hydroxyl radicals (OH-), prevent
the propagation process so that free radicals will not
react with oxygen and automatically does not
directly reduce MDA levels in rat brain.
This is also strengthened by the presence of other
bioactive compounds including flavonoids where
these compounds can ward off free radicals by
reducing free radical compounds so that they
become stable compounds. Binding of free radicals
by flavonoids will prevent chain radical reactions
that damage protein function and normal tissue
structure. Flavonoids are compounds that can be
easily modified to stop radicals so that they can
prevent oxidative stress in cells and increase the
enzyme protease in tissues
.
3.2 Effect
of Mangosteen Skin Extract
Therapy on TBI Model Blood Serum
Protease Activity
Protease activity is the ability of proteases to
hydrolyze peptide bonds in proteins. The method
used in the analysis of protease activity is
spectrophotometry. The method is based on
enzymatic hydrolysis by proteases tested from a
casein substrate Solution at pH 7, and followed by
deposition of non-hydrolyzed substrates using
trichloroacetic acid (TCA) 4% to stop the reaction.
The result of hydrolysis from the casein substrate
Solution is L-tyrosine. Therefore, in the
measurement of protease activity, the tyrosine
standard curve is used with λ = 275 nm because the
final product (product) is measured by UV
spectrophotometer at a maximum wavelength of 275
nm. The protease activity unit of rat blood serum
(Rattus norvegicus) is defined as the number of
tyrosine units produced by hydrolysis of peptide
bonds in proteins by protease isolated from rat
duodenum (Rattus norvegicus) under optimum
conditions in pH 6.5, temperature 370 C, and time
incubation of 60 minutes.On the tyrosine standard
curve obtained by the equation of the line y=0.015x
- 0.013. The line equation is used to calculate the
measured tyrosine concentration from the study so
that the protease activity can be
calculated for each
treatment. A complete calculation of protease activity
is presented in Attachment 9.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
138

4 CONCLUSIONS
Based on the results of research that has been done
can be concluded that:
1. Mangosteen skin extract therapy can repair
damage to the cortex of the rat brain.
2. Mangosteen peel extract therapy can reduce
malondialdehyde (MDA) expression in rat brain
by 2.16 ± 0.37.
3. Mangosteen peel extract therapy can reduce the
activity of protease enzymes by 47.62%.
ACKNOWLEDGEMENT
We would like to thanks to Prof. Dr. drh.
Aulanni’am., DESS and Prof Dr. Ir Chanif Mahdi.,
MS who gave us a research, , thus we can write a
paper based on several study cases.
REFERENCES
Warpani, S.P. (2002), Pengelolaan Lalu Lintas dan
Angkuatan Jalan. Penerbit ITB. Bandung
Armitage, D. (2004). Rattus norvegicus. Animal Diversity
Web. University of Michigan of Zoology
Miryanti, Y.I.P.A., Sapei, L., Budiono, K. & Indra, S.
(2011). Ektraksi Antioksidandari Kulit Buah Manggis
(Garcianamangostana L.). LPPM UniversitasKatolik
Parahyangan. Bandung
Murfu’ati N.,Sarjadi,Winarto, & Djamiatun, K. (2014).
Efek ektrak Kulit Manggis terhadap Ekspresi Protein
Bcl-2 dan Jumlah Sel Mati Tubulus Ginjal Tikus yang
Diinduksi Formalin. J. Kedokteran Brawijaya, 28(2).
Fakultas Kedokteran. Universitas Muhammdiyah
Semarang
Yigit, S., Yurdakok, M., & Oran, O. (1999). Serum
Malondialdehyde Concentration in Babies with Hyper
bilirubinaemia.Arch. Dis. Child. Fetal Neonatal Ed.
80:235-7
Hantoko, S. & Drajat, R.S. (2003). ManfaatPemberian
Gelombang Ultrasonik Intesitas Rendah untuk
Mempercepat Pembentukan Kalus Fraktur Tibia. Maj.
Kedok. Unibraw,19(2) : 8-13
Mansour, N. A., Aulanni’am, A. & Kusnadi J. (2013).
Garcina mangostana Linn. Pericarp Extract Reduced
Malondialdehyde (MDA) Level in Cigarette Smoke
Exposed Rats. Internasional Refereed Journal of
Engineering and Science (IRJES). Vol PP.01—05
Yatman E. 2012. Kulit Buah Manggis Mengandung
Xanton yang Berkhasiat Tinggi. Universitas
Borobudur
Sato I, Kurihara Ando N, Kota, K., Iwaku, K. & Hosino,
E. (1996). Sterilization ofinfected root-canal dentine
by topical application of a mixture of ciprofloxacin,
metronidazole and minocycline in situ. Journal
ofInternational Endodontic. 29, 118-124
Reed, C.E. & Kita, H. (2004). The Role of Protease
Activation of Inflamation in Allergic Respiratory
Disease. Journal Allergy Clinical Immunology.
114(5):997-1008
A., Polidori, C., Bedetti, C., Ercolani, S., Senin, U.&
Mecocci P. 1999, Assosiation Between Ischemic
Stroke and Increased Oxidative Stress. Perugia
Block, G., Dietrich, M., Norkus, E.P., Morrow, J.D.,
Hudes, M & Caan, B. (2002). Factor Associated with
Oxidative Stress in Human Populations. Am J
Epidemiol156 : 274 – 85
Al-Ashy, R., et al. (2006). The role of NF-κB inmediating
the anti-inflammatory effects of IL-10 in intestinal
epithelial cells. Cytokine. 36(1): p. 1-8
Bodger, K., et al. (2001). Interleukin 10 in
Helicobacterpyloriassociated gastritis:
immunohistochemical localisation and in vitro effects
on cytokine secretion. Journal of clinicalpathology.
54(4): p. 285-292
Bizaliel, E. (2011). Pemberian Ektrak Metanol Daun Kelor
(Moringa Oleifera) Terhadap Kadar Malondialdehida
(MDA) Pada Kolon Tikus Wistar yang Diinduksi
DMBA 7,12 Dimethylbenz Anthracene,Skripsi,
FakultasKedokteran, Universitas Brawijaya, Malang
Taylor C.R., Shan, R.S., & Barr, N.J. (2010). Techniques
Of Immunohistochemistry: Principles, Pitfalls,
andStandardization.In:
DabDJ,editor.Diagnosticimmunohistochemistry.3rd
ed.Philadelphia: Saunders-Elsevier Inc
Aslanian, F.M.N.P., Noe R.A.M., Antelo, D.P., Farlas,
R.E., Das P.K., Galadari, I.
(2008).Immunohistochemical Fndings
inActiveVitiligoiIncludingDepigmentating Lesions
and Nonlesional Skin. The OpenDermatol J. 2:105-10.
Elwan, N.M., El-Ashmawy, A.A., Gheida, S.F. &
Rizk,O.K.(2013).ImmunohistochemicalExpression of
c-kit Receptor (CD117) in Two Pigmentary Disorders.
J Clin Exp Dermatol Res.4:190. doi:10.4172/2155-
9554.1000190.
Harjadi. (1990). Ilmu Kimia Analitik Dasar. Jakarta: PT.
Gramedia.
Vermes, I., Haanen, C., & Reutelingsperger, C., 2000,
Flow Cytometry of ApoptoticCell Death. Journal of
ImmunologicalMethods. 243, 167-190.
Redha, A. (2010). Flavonoid: Struktur, Sifat Antioksidatif
dan Peranannya dalam Sistem biologis.
JurusanTeknologi Pertanian Politeknik Negeri
Pontianak. Jurnal Belian, 9(2) : 196-202
Simamora, A. (2009). Flavonoid dalam Apel danAktivitas
Antioksidannya.Universitas Kristen Krida Wacana,
Jakarta.
Winarsi,H. (2007). Antioksidan Alami danRadikal Bebas.
Penerbit Kanisius.Yogyakarta
Bagchi, K. & Puri, S. (1998). Free Radicaland Antioxidant
in Health and Disease. Eastern MediterraneanHealth
Journal 4 (2), 350-360
Effect of Therapy on Mangosteen (Garcinia mangostana L.) Bark Extract on Serum Blood Blood Protease Activity and Expression of
Malondialdehyde (MDA) on Rattus norvegicus Traumatic Brain Injury Model
139

Evans, C.A.R., Diplock, A.T., & Simons, M.C.R. (1991).
Techniques in FreeRadical Research, Elsevier
SciencePublishers BV, Amsterdam, 1-50, 125-149
Hsieh, Y., Chang, C. d&Lin, C. (2006).
SeminalMalondialdehyde Concentration but not
Glutathione Peroxidase Activity is Negatively
Correlated with Seminal Concentration and Motility.
Int J Biol Sci, 21, 23-29
Tewtrakul S., Wattanapiromsakul, C.& Mahabusarakam,
W. (2009). Effect ofCompound from Garcina
Mangostana on inflammatory Mediator in
RAW264.macropaphage cells. J Ethnopharco,
121:379-382
Weecharangsan, W. P., Opanasopit, M., Sukma, T.,
Ngawhirunpat, U.,Sotanaphunand P. &
Siripong.(2006). Antioxidative and Neuroprotective
Activities of Extracts from The Fruit Hull of
Mangosteen (Garcina mangostana Linn). 15(4):281-
287
Paramawati., R. (2010). Dahsyatnya Manggisuntuk
Menumpas Penyakit. Agromedia Pustaka Jakarta
Akhdiya, A. (2003). Isolasi Bakteri Penghasil Enzim
Protease Alkalin Termostabil. Buletin Plasma Nutfah.
9(2): 38-44
Kosim M. & Putra, S.R. (2010). Pengaruh Suhu pada
Protease dari Bacillus subtilis. Tugas Akhir, Jurusan
Kimia, ITS, Surabaya
Redha, A. (2010). Flavonoid: Struktur, Sifat
Antioksidatif dan Peranannya dalam Sistem Biologis.
JurusanTeknologi Pertanian Politeknik Negeri
Pontianak. Jurnal Belian, 9(2): 196-202
Danihelova, M., Veverka, M. & Sturdik, E. (2013)
Inhibition of Pathophysyiological Proteases with
Novel Quercetin Derivates. SlovakUniversity of
Technology, Slovakia. Journal Acta Chimica Slovaca,
6(1):115-122
Bendo, A.A. & Sakabe T. (2007). Anesthethic
Management of Head Trauma, Edisike 4. New York :
Lippincott Williams-Wilkins
Won, S.J., Kim, D.Y., Gwang, B.J. (2002). Cellular and
Molecular Pathway of Ischemic Neuronal Death. J
BiochemMolBiol
Werner, C. & Engelhard,K.(2007). Pathophysiology of
Traumatic BrainInjury.Br. J Anesth
Tahir S. & Shuja A. (2011). Head Injury Pathology.
Dalam: Independent Review, SurgicalPrinciple.
Edisi ke-85. Pakistan: Faisalabad; 2011
Mauritz , W., Wilbacher, I. & Majdan, M.. (2008).
Epidemiology, Treatment andOutcome of Patients
after Severe TraumaticBrai Injuryin European Regions
with Different Economic Status. The EuropeanJournal
of Public Health.
Tony, K., (2003). HeadTraumas-Comparative
Imaging Component, Lecture note. Medical
ImagingScience 335. Perth Australia:
CurtinUniversity of Technology.
Thompson, H.J., McCormick, W.C. & Kagan, S.H.
(2006). Traumatic Brain Injury inOlder
Adults:Epidemiology,Outcomes and Future
Implications. J Am Geriatri Soc
Chaudhry, I.B., Hallak, J., Husain, N., Minhas, F.A.,
Stirling, J., Richardson, P., Dursun, S., Dunn, G. &
Deakin, B. 2012. Minocycline Benefits Negative
Symptoms in Early Schizophrenia: a Randomized
Double-Blind Placebo Controlled Clinical Trial in
Patients on Standard Treatment. Journal
ofPsychopharmacology
Yong, V.W., Wells, J., Giuliani, F., Casha, S., Power, C.
& Metz, L.M. (2004) The Promise of Minocycline in
Neurology. The Lancet Neurology
Miyaoka, T., Yasukawa, R., Yasuda, H., Hayashida, M.,
Inagaki, T. & Horiguchi, J. (2008). Minocycline
asAdjunctive Therapy for Schizophrenia. An open-
labelstudy: Clinical Neuropharmacology.
Colovic, M. &Caccia, S. (2003). Liquid Chromatographic
Determination of Minocycline in Brain-to-Plasma
Distribution Studies in The Rat. Journal of
Chromatography
Del L.A.R, Sandalio, L.M., Corpas, F.J., Palma, J.M. and
Barroso, J.B. (2006). Reactive Oxygen Species and
Reactive NitrogenSpecies in Peroxisomes.
Production,scavenging, and role in cell signaling.
Plant Physiology.
Taniyama Y and Griendling K. (2003). Reactive Oxygen
Species in The Vasculature.Molecular and
CellularMechanisms. J.Hypertension
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
140
