
Interventions of Cetrorelix Acetate in Estrogen Beta Receptor
Expression and Histopathology in Rats Oviduct
Herlina Pratiwi
1
, Aulia Firmawati
2
, Diana Rahmayani Putri
3
, Albiruni Haryo
4
, Analis Wisnu
Wardhana
5
1
Embryology Laboratory, Faculty of Veterinary Medicine, Brawijaya University
2
Reproduction Laboratory, Faculty of Veterinary Medicine, Brawijaya University
3
Graduate Student of Veterinary Medicine, Faculty of Veterinary Medicine, Brawijaya University
4
Department of Pathology, Faculty of Veterinary Medicine, Brawijaya University
5
Department of Anatomy and Histology, Faculty of Veterinary Medicine, Brawijaya University
Keywords: Rat Ovarian Hypofunction, Cetrorelix Acetate, Estrogen Receptors, Oviduct Cilia.
Abstract: Ovarian hypofunction is pathologic conditions where is the ovary being abnormal. The abnormality of the
ovary can be induced by the abnormality of the endocrine that regulates the development of the ovary such
as, follicle-stimulating hormone (FSH) and luteinizing hormone LH. The production of FSH and LH in the
pituitary is determined with the Gonadotropin hormone (GnRH) stimulation. Development of rat ovarian
hypofunction models can be performed with the induction of cetrorelix acetate which has an antagonist
effect of GnRH. This research was conduct to know the effect of induction of cetrorelix acetate on rat
oviduct estrogen beta receptor expression and histopathology. The study used three groups of female rats
(Wistar strain) 8-10 weeks old and 150-180 gram weight, each group consisting of six rats. The first group
(control) without cetrorelix acetate, the second group treated with cetrorelix acetate 0.009 mg/kg BW and
the third group treated with cetrorelix acetate 0.0135 mg/kg BW. Observations of estrogen beta receptor
expressions (ERs β) are carried out with immunochemical methods, while observations of histopathological
changes of oviduct carried out by Hematocsilin-Eosin (HE stain). The results obtained indicate a significant
difference from the administration of the GnRH antagonists in the three treatment groups, among others, the
largest reduction of the expression of the estrogen receptor of the ES β by 59.2%, as well as the thinning of
the fallopian tubes and the reduced cilia. The conclusion of the study was Cetrorelix acetate as a GnRH
antagonist capable of lowering the beta estrogen receptor expression and reducing the number of cilia as
well as the viscosity of the wall lining of the fallopian tubes.
1 INTRODUCTION
The consumption need of protein from animals
source especially for meat per capita in Indonesia for
one day reaches 3.35 grams or 5.91%. This needs
every year increases. The largest increase in
consumption of livestock products experienced was
in 2016 about 32.17% (Ditjen PKH, 2017). The
increasing number of consumption of livestock
products, especially meat, can cause an increase in
import activities. One of the causes of the increase in
beef import activities in Indonesia is the low
reproduction performance of female cows in
Indonesia and reproductive disorders. One of the
most common examples of reproductive disorders in
female cows is ovarian hypofunction.
Sutiyono et al. (2017) explain that ovarian
hypofunction is a decrease in ovarian activity in
producing oosit or ovum. Ovarian hypofunction is a
pathologic condition caused by impaired secretion of
follicle-stimulating hormone (FSH) and luteinizing
hormone (LH) (Hermadi, 2015). Impaired secretion
of FSH and LH can be caused by decreased
secretion of Gonadotropin-Releasing Hormone
(GnRH) by the hypothalamus. According to
Wulandari (2013), GnRH functions to stimulate
FSH and LH secretion. FSH functions are to
stimulate follicular development and estrogen
secretion, while LH functions for the maturation of
de Graaf follicle and ovulation. The development
and function of the reproductive organs are highly
dependent on the secretion of FSH and LH in the
62
Pratiwi, H., Firmawati, A., Putri, D., Haryo, A. and Wardhana, A.
Interventions of Cetrorelix Acetate in Estrogen Beta Receptor Expression and Histopathology in Rats Oviduct.
DOI: 10.5220/0009587300620067
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 62-67
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

anterior pituitary controlled by GnRH in the
hypothalamus. The low secretion of GnRH in the
hypothalamus and low secretion of FSH-LH in the
anterior pituitary will cause anestrus animals
(Pemayun, 2010). One of the medicines that have an
effect on GnRH antagonists is Cetrorelix acetate.
This GnRH antagonist can cause a decrease in
ovarian function by suppressing binding between
GnRH and its receptors so it can inhibit the
synthesis of Follicle Stimulating Hormone (FSH)
and Luteinizing Hormone (LH) (Wen et al., 2010).
The inhibition of FSH and LH synthesis causes
inhibition of folliculogenesis (Sharif et al., 2016).
Delay in folliculogenesis can cause inhibition of
estrogen hormone synthesis which results in
decreased expression of estrogen receptors in tissues
(Caldon, 2014).
Estrogen is a steroid hormone that has functions
in many tissues in the body, including the oviduct.
The primary function of estrogen is for tissue
proliferation of the reproductive organs and other
tissues related to the reproductive system. The
morphology of oviduct epithelial cells is influenced
by ovarian hormones, one of which is estrogen
(Crow et al., 1994). GnRH antagonists that inhibit
estrogen synthesis cause a decrease in the bonds
between estrogens and its receptors, so it will make
the receptors inactive and not expressed. According
to Trisunuwati (2016), estrogens need estrogen
receptors to carry out their functions. In the oviduct,
estrogen hormone activity requires binding with
receptors to stimulate epithelial cell proliferation, so
the absence of estrogen receptors in the fallopian
tubes can cause inhibition of ciliary formation in the
fallopian tubes.
2 MATERIALS AND METHOD
The tools used include terumo® 1 cc syringes,
terumo® 3 cc syringes, blades, surgical scissors,
anatomical tweezers, serological tweezers, surgical
boards, Petri dishes, and pins, microtomes,
incubators, and optilab microscopes.
Materials used include rabbit feed (pellets) SP®,
husks, and sufficient water, Phosphate Buffer Saline
(PBS), formaldehyde, alcohol, xylol, 0.9%
physiological NaCl, paraffin, Hematoxillin-Eosin
stain, entellan, primary antibody ERS β brand
abcam® (ab288), secondary antibody labeled
peroxidase, normal Horse serum 2.5% brand
abcam® (ab7484), hydrogen peroxide, methanol and
chromogen diaminobenzidine tetrahydrochloride
(DAB) brand abcam® (ab64238).
The rats were acclimatized for 7 days for
adaptions, given rabbit feed and drinking water in an
adlibitum. The group was divided into 3 groups
which included a control group without
administration of Cetrorelix acetate, the first
treatment group (P1) with the administration of
0.009 mg/kg BW Cetrorelix acetate and the second
treatment group (P2) with 0.0135 mg/kg BW of
Cetrorelix acetate.
The vaginal swab preparations carried out by
dipping the cotton bud in physiological NaCl than
the rat placed in a dorsal lying position. The vaginal
swab done by inserting a cotton bud in the vagina by
rotating 360
o
, then cotton bud removed and swab on
the slide. The slide allowed to dry and then fixed
using alcohol. The preparations that have been fixed
with alcohol and which have dried then are stained
with Eosin Negrosin for 15 minutes, then rinsed
with running water with a small flow of water and
rinsed slowly. The results of vaginal swabs are
observed under a microscope with a magnification
of 100x and 400x to see vaginal cells. The vaginal
swab was carried out before injected with the
cetrorelix acetate to equalize the estrous cycle of the
rat.
After the treatments, the rat was euatanated with
cervical dislocation method (University of
Melbourne Ethics and Animal Welfare Commission,
2016). The rat is vertically opened from the posterior
abdomen to the thorax cavity. The oviduct organs
are taken, then washed with physiological NaCl and
collected in pots containing 10% Formaldehyde.
The ovary is processed to block paraffin and then
continue with Haemotoxyline-eosin and
immunohistochemical stain. The changes in the
histopathological of the oviduct observed with the
number of cilia and the thickness of the fallopian
tube wall. The expression estrogen beta receptor was
carried out at 40 times magnification and 5 visual
fields (Le, 2005 and Okada et al., 2004). Estrogen
receptors in the oviduct observed in all layers of
cells. Expression of the estrogen beta receptor
observed by calculating the average number of
expressed cells using the ImmunoRatio application.
3 RESULT
The estrogen beta receptor expression in the oviduct
observed with immunohistochemical methods
(Table 1). This method will give a brown color to
the target cell. These receptor expressions vary
according to the stage of the ovarian cycle and peak
in the middle of the cycle (Amso et al., 1994; Pollow
Interventions of Cetrorelix Acetate in Estrogen Beta Receptor Expression and Histopathology in Rats Oviduct
63
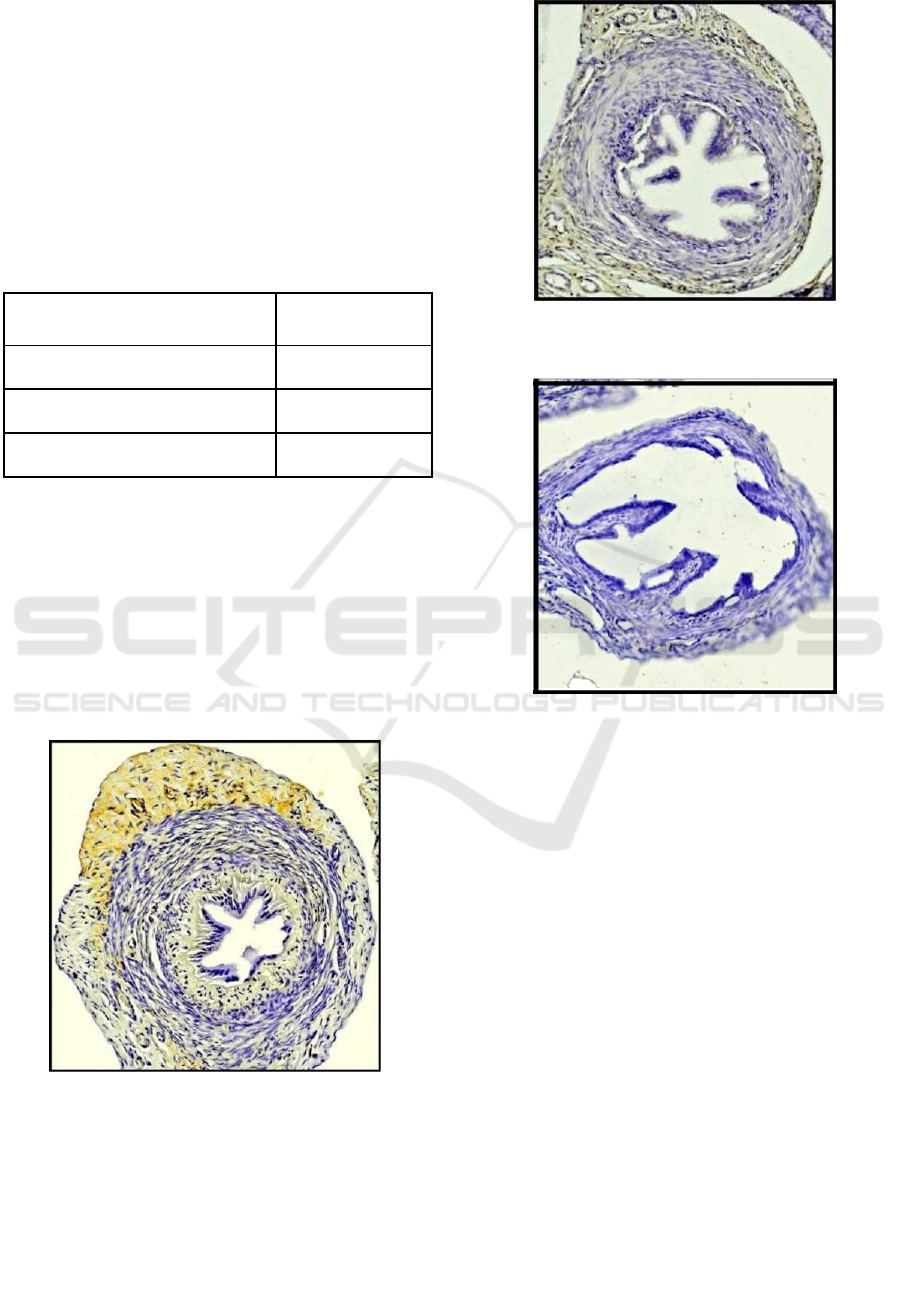
et al., 1981). Estrogen hormones in the oviduct are
involved in the regulation of oviduct functions
themselves, such as oviduct fluid formation and
gamete transport (McDonell et al., 2002).
Functionally, estrogen beta receptor (ER-β)
functions in the growth of oviduct, regulation of
protein content and expression of growth factors.
Whereas ER-β functions in the process of gamete
transportation.
Table 1. Expression of ERs β in rat injected with cetrorelix
acetate
Group Average ERs β
expression (%)
Control group
54,01
c
P1 group
32,30
b
P2 group
22,03
a
Note: a, b, c notations indicate a significant difference
between one treatment and another.
Based on the estrogen beta receptor expression in
the table above, it can be seen that the average ERs β
expression in the treatment group (P1 and P2)
decreased compared to the control group. This data
also showed that the higher dose of cetrorelix acetate
has lowest expression of ERs β in rat. The
expression of β ERs can be seen in the Figure 1, 2
amd 3 below.
Figure 1. Expression of β ERs in rat oviduct of control
group (40 times magnification, cross section).
Figure 2. Expression of β ERs in rat oviduct of P1 group
(40 times magnification, cross section).
Figure 3. Expression of β ERs in rat oviduct of P2 group
(40 times magnification, cross-section).
The results of the vaginal swab that conducted
before euthanasia, showed that the rat was in the
metestrus phase. The oviduct in the metestrus phase
characterized by the thickening of the oviduct wall
and the formation of cilia (Restall, 1966). An
increasing number of ciliated cells and thick
fallopian mucosal layers occur in the estrous and
metestrus phases. Increased thickness of the
fallopian tube lining is caused by an increase in the
number of ciliated cells and secretory cells (Liputo,
2006). At the end of the metestrus phase, non-ciliary
epithelial cells and ciliated epithelial cells undergo
apoptosis, but most of the secretory granules remain
in the secretory cells which then revert to structural
changes and begin the process of ciliogenesis in the
next phase (Kress and Morson, 2007).
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
64
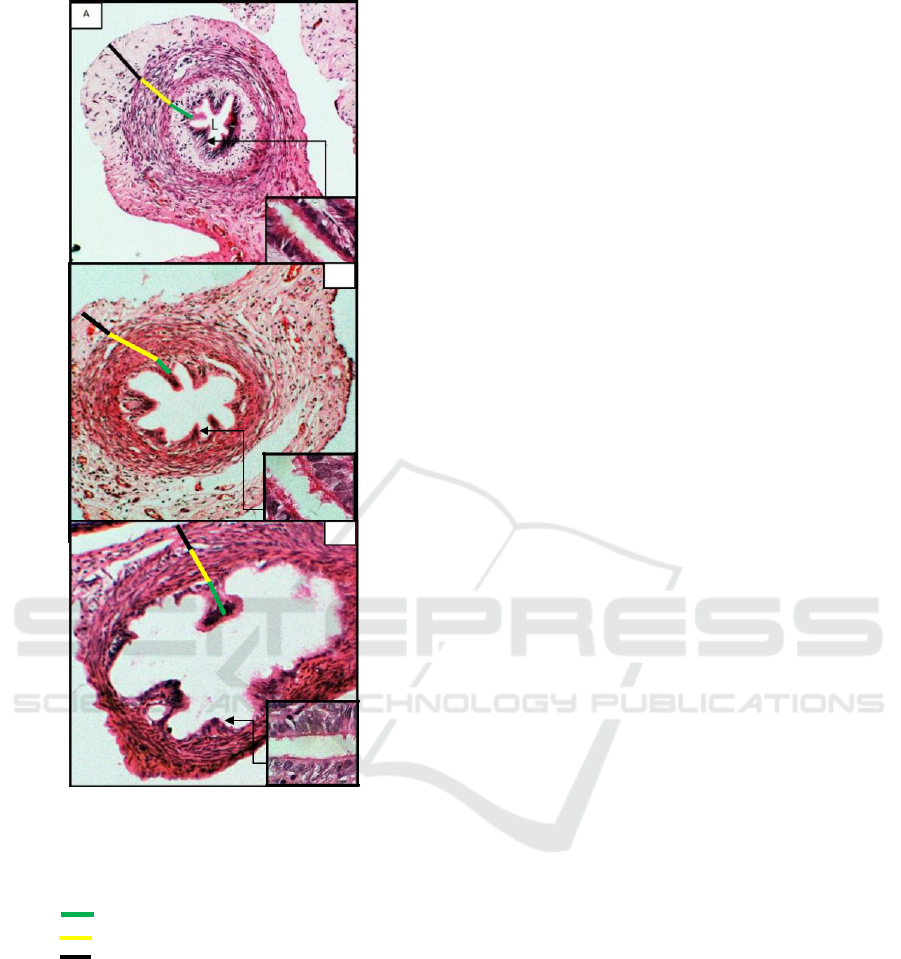
B
L
C
L
Figure 4. Histophatology of rat oviduct induced by
cetrorelix acetate, A. Control group, B. P1 group, and C.
P2 group. Haemotoxylin-eosin stain, 40 times
magnification.
Note: tunica adventitia (Serosa);
tunica media
(Muscularis); tunica
intima (Mucosa);
(L) : lumen
The control group showed thicken wall and
many cilia (Figure 4.A), wich group P1 and P2
reduce in-wall thick and the number of cilia in the
oviduct (Figure 4. B and C).
4 DISCUSSION
The expression of ER β decreased in the treatment
group P1 injected Cetrorelix acetate 0.009 mg/kg
BW and P2 injected Cetrorelix acetate 0.0135 mg/kg
BW. Decreased expression of β estrogen receptors
caused by injection of Cetrorelix acetate as
a Gonadotropin-Releasing Hormone (GnRH)
antagonist occurs because the cetrorelix acetate
competes with GnRH to bind to membrane receptors
on pituitary cells and control the release of Follicle
Stimulating Hormone (FSH) and Luteinizing
Hormone (LH), thereby delaying the LH surge and
ovulation (Rodney, 2013). Induction of Cetrorelix
acetate which is a GnRH antagonist causes
inhibition of estrogen synthesis that occurs during
the process of folliculogenesis it makes a decrease of
estrogen production. This decrease causes a
decreased binding of estrogen and its receptor so the
receptors are inactive and not expressed. These
results can be concluded that the injection of
Cetrorelix acetate can decrease the estrogen receptor
expression in the oviduct.
The reduced number of cilia and the thickness of
the oviduct wall caused by the injected by Cetrorelix
acetate, it caused by the suppressed secretion of
Follicle Stimulating Hormone (FSH), Luteinizing
Hormone (LH) and estrogen (Griesinger et al.,
2005). Decreased levels of FSH and LH can inhibit
folliculogenesis and ovulation (Cooke et al., 1998;
Hafizuddin et al., 2012). Inhibited folliculogenesis
by injection of cetrorelix acetate can cause impaired
estrogen hormone synthesis. Impaired synthesis of
estrogen hormones can affect the development of
reproductive organs including oviduct wall.
The function of estrogen is for epithelial cell
proliferation, secretion, and ciliogenesis (Verhage et
al., 1979; Donnez et al., 1985). Ciliogenic activity
and secretion are caused by estrogen which acts
through estrogen receptors on oviduct epithelial cells
(Lauschová, 1999; Listy and Chakravarti, 2011).
The mechanism of the hormone estrogen which can
affect the thickness of the oviduct can be explained
through estrogen activity in the cells making up the
oviduct. Estrogen activity in cells begins after
estrogen bonds in the cytosol. The estrogen and
receptor complex further diffuses into the cell
nucleus and attaches to DNA. The estrogen-receptor
complex binding with DNA induces the synthesis
and expression of mRNA in the form of protein
synthesis thereby increasing target cell activity,
which is indicated by cell proliferation (Johnson and
Everitt, 1984).
Increased thickness of the oviduct mucosal wall
caused by an increase in the number of cells making
up the fallopian tissue. Increasing the number of
cells both secretory epithelial cells and ciliary
epithelium can cause the oviduct mucosal layer to
Interventions of Cetrorelix Acetate in Estrogen Beta Receptor Expression and Histopathology in Rats Oviduct
65

get thicker. The thickness of the oviduct mucosal
wall affects individual fertility (Umami et. Al,
2014). This is consistent with the statement of Crow
et al. (1994) regarding the morphology of oviduct
epithelial cells affected by estrogen, which act
through their receptors, where the hormone estrogen
causes mucosal glandular tissue to proliferate and
increases the number of ciliated epithelial cells.
5 CONCLUSION
Injection of Cetrorelix acetate as GnRH antagonist
in the rat can reduce the expression of estrogen beta
receptor, decrease wall thickness and cilia in rat
oviduct with the best doses was 0.0135 mg/kg BW.
ACKNOWLEDGMENTS
We many thanks to LPPM Universitas Brawijaya
and Faculty of Veterinary Medicine, Universitas
Brawijaya for providing research assistance funds
and supporting the completion of this research.
REFERENCES
Amso N. N., Crow J., and Shaw R. W. 1994. Comparative
Immunohistochemical Study of Oestrogen and
Progesteron Receptors in the Fallopian Tube and
Uterus at Different Stages of the Menstrual Cycle and
the Menopause. Human Reproduction Vol. 9 No. 6:
1027-1037. Oxford University Press.
Animal Welfare and Ethics Committee. 2016. Humane
Killing of Mice and Rats. University of Melbourne.
Caldon, C. E. 2014. Estrogen Signaling and the DNA
Damage Response in Hormone Dependent Breast
Cancers. Front Oncol, 4106-114.
Cooke P. S., Buchanan D. L., Lubahn D. B., and Cunha G.
R. 1998. Mechanism of Estrogen Action: Lessons
from the Estrogen Receptor alpha Knockout Mouse.
Biology of Reproduction Vol. 59 Issue 3: 470-475.
Crow J., Nazar N. A., Lewin J., and Shaw Robert W.
1994. Morphology and Infrastructure of Fallopian
Tube Epithelium at Different Stages of the Menstrual
Cycle and Menopause. Human Reproduction Vol. 9
No. 12: 2224-2233. Oxford University Press.
Daftary, Shirish and Chakravarti, Sudip. 2011. Manual of
Obstetrics 3rd Edition. Elsevier India.
Direktorat Jenderal Peternakan dan Kesehatan Hewan.
2017. Statistik Peternakan dan Kesehatan Hewan.
Kementerian Pertanian Republik Indonesia.
Donnez J., Casanas-Roux F., Caprasse J., Ferin J., and
Thomas K. 1985. Cyclic Changes in Ciliation, Cell
Height and Mitotic Activity in Human Tubal
Epithelium during Reproductive Life. Fertility and
Sterility Vol. 43 No. 4. The American Fertility
Society.
Griesinger G., Diedrich K., Devroey P., and Kolibianakis
E. M. 2005. GnRH Agonist for Triggering Final
Oocyte Maturation in the GnRH Antagonist Ovarian
Hyperstimulation Protocol: A Systematic Review and
Meta-Analysis. Human Reproduction Update Vol. 12
No. 2: 159-168. Oxford University Press.
Hafizuddin, Siregar T. N., and Akmal M. 2012. Hormon
dan Perannya dalam Dinamika Folikuler pada Hewan
Domestik. Jurnal Edukasi dan Sains Biologi Vol. 1
No. 1. Al-Muslim University.
Hermadi, Herry Agoes. 2015. Pemberantasan Kasus
Kemajiran pada Ternak Menuju Kemandirian di
Bidang Kesehatan Reproduksi Hewan dan Ketahanan
Pangan di Indonesia. Universitas Airlangga Press.
Hutabarat, Mitra Artha Kurnia. 2019. Pengaruh GnRH
Antagonis terhadap Ekspresi Reseptor Estrogen (ERs)
dan Perubahan Histopatologi Ovarium pada Tikus
Putih (Rattus norvegicus). Skripsi. Universitas
Brawijaya. Malang.
Johnson, M. H. and Everitt, B. J. 1984. Essential
Reproduction. Edisi ke-2. London: Blackwell
Scientific Publications.
Kress, Annetrudi and Morson, Gianni. 2007. Changes in
the Ovidcuctal Epithelium during the Estrous Cycle in
the Marsupial Monodelphis domestica. Journal
Anatomical Society of Great Britain and Ireland.
Lauschová, I. 1999. Influence of Estrogen and Progesteron
on Ultrastructural Indices of Oviductal Epithelieum in
Sexually Immature Mice. Acta Vet. Brno 68: 13-21.
Department of Histology and Embryology, Masaryk
University, Czech Republic.
Le Loıc Marchand, Timothy Donlon, Laurence N.
Kolonel, Brian E. Henderson, and Lynne R. Wilkens.
2005. Estrogen Metabolism– Related Genes and
Breast Cancer Risk: The Multiethnic Cohort Study.
Cancer Epidemiology Biomarkers Previews Vol. 14
No. 8.
Liputo, Khaeruni Putriani. 2006. Studi Histopatologi
Pengaruh Pajanan Asap Rokok Kretek terhadap Organ
Reproduksi Betina Tikus Putih (Rattus norvegicus).
Institut Pertanian Bogor: Fakultas Kedokteran Hewan.
McDonell, Donald P. and Norris, John D. 2002.
Connections and Regulation of the Human Estrogen
Receptor. Science Vol. 296. Department of
Pharmacology and Cancer Biology, Duke University
Medical Center.
Okada A., Ohta Y., Brody S. L., Watanabe H., Krust A.,
Chambon P., and Iguchi T. 2004. Role of foxj1 and
Estrogen Receptor Alpha in Ciliated Epithelial Cell
Differentiation of The Neonatal Oviduct. Journal of
Molecular Endocrinology Vol. 32: 615- 625. Society
of Endocrinology.
Pemayun, Tjok Gde Oka. 2010. Kadar Progesteron Akibat
Pemberian PMSG dan GnRH pada Sapi Perah yang
Mengalami Anestrus Postpartum. Buletin Veteriner
Udayana Vol. 2 No. 2: 85-91. ISSN: 2085-2495.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
66

Pollow K., Inthraphuvasak J., Manz B., Grill H., and
Pollow B. 1981. A Comparison of Cytoplasmic and
Nuclear Estradiol and Progesterone Receptors in
Human Fallopian Tube and Endometrial Tissue.
Fertility and Sterility Vol. 36 No. 5. The American
Fertility Society.
Restall, B. J. 1966. Histological Observations on the
Reproductive Tract of the Ewe. Australian Journal of
Biological Sciences Vol 19: 673-686.
Rodney, J. Y. 2013. Biotechnology and
Biopharmaceuticals: Transforming Proteins and Genes
into Drugs, Second Edition. John Wiley & Sons, Inc.
Sutiyono, Daud Samsudewa, and Alam Suryawijaya.
2017. Identifikasi Gangguan Reproduksi Sapi Betina
di Peternakan Rakyat. Jurnal Veteriner Vol. 18, No. 4.
Syarif Rul A., Soejono Sri K., Meiyanto E., and
Wahyuningsih M. S. H. 2016. Efek Kurkumin
terhadap Sekresi Estrogen dan Ekspresi Reseptor
Estrogen β Kultus Sel Granulosa Babi Folikel Sedang.
Jurnal Kedokteran Brawijaya Vol. 29 No.1.
Yogyakarta: Universitas Gadjah Mada.
Trisunuwati, Pratiwi. 2016V. The Role of Leaf Water
Clover (Marsilia crenata) Squeeze towards Estrogen
Blood Level and Uterine Histology in Rats (Rattus
norvegicus). Jurnal Ternak Tropika Vol. 17 No. 2: 1-7.
Malang: Universitas Brawijaya.
Umami, Riza, D Pande Made., Winarsih Sri., 2014.
Pengaruh Vitamin C dan E terhadap Histologi Oviduk
pada Tikus yang dipapar MSG. Jurnal Kedokteran
Brawijaya. Vol.28. No. 2.
Verhage H. G., Bareither M. L., Jaffe R. C., and Akbar M.
1979. Cyclic Changes in Ciliation, Secretion and Cell
Height of the Oviductal Epithelium in Women.
Journal of Anatomy Vol. 156 No. 4: 505-521.
Wen J., Feng Y., Bjorklund C. C., Wang M., Orlowski R.
Z., Shi Z., Liao B., O’Hare J., Schally A. V., and
Chang C. 2010. Luteinizing Hormone-Releasing
Hormone (LHRH)-I Antagonist Cetrorelix Inhibits
Myeloma Cell Growth In vitro and In Vivo. Molecular
Cancel Therapeutics Vol. 10 No. 1. American
Association for Cancer Research.
Wulandari, E. and Hapsari Ayu, F. R. 2013. Buku Peran
Hormon Sebagai Regulator Fungsi Organ. Fakultas
Kedokteran dan Ilmu Kesehatan. Jakarta: UIN Jakarta
Press.
Interventions of Cetrorelix Acetate in Estrogen Beta Receptor Expression and Histopathology in Rats Oviduct
67
