
Study of Oviduct Expression Specificct Glycoprotein1 (OVGP1) on
Oocyte and Goat Follicles (Capra hircus)
Aulia Firmawati
1
, Herawati
2
, Herlina Pratiwi
3
, Latiffatul ‘Ainiyah
4
, and Regi Abdul Rozzaaq A. S.
4
1
Reproduction Laboratory, Faculty of Veterinary Medicine, Brawijaya University, Indonesia
2
Public Health Laboratory, Faculty of Veterinary Medicine, Brawijaya University, Indonesia
3
Anatomy Laboratory, Faculty of Veterinary Medicine, Brawijaya University, Indonesia
4
Undergraduate Bachelor Student, Faculty of Veterinary Medicine, Brawijaya University, Indonesia
Keywords: OVGP1, Liquid Semen, Goat, Membrane Integrity, Motility
Abstract: Oviduct Specific Glycoprotein (OVGP1) is a glycoprotein that has been identified as a protein secreted from
unciliated secretory epithelial cells in oviducts with a molecular weight of 65 kDa in goats. The expression of
this protein is very dependent on estrogen levels and the oestrus phase of the species. On the other hand,
glycoprotein plays an important role in the process of oocyte maturation, spermatozoa capitation, and early
embryonic development. The purpose of this research was to find the location of OVGP1 expression in
oocytes and follicles of kacang goat (Capra hircus), an endogenous goat from Indonesian . The methods used
in this study are: immunocytochemical techniques to see OVGP1 expression on oocytes and on goat follicles.
OVGP1 expression in goat oocytes (Capra hircus) observed using immunocytochemical techniques was
detected in the cumulus ooporus section, the zona pellucida, perivitelline space, and plasma oocyte membrane,
whereas in the follicle the OVGP1 expression observed using immunohistochemical methods showed that
OVGP1 was expressed in granulosa cells, external and internal theca cells.
1 INTRODUCTION
Polyspermia is the process of fusion of eggs by two
or more consecutive spermatozoa (polyspermy) is a
lethal condition in most organisms (Frank, 2000).
Polyspermi, the most common abnormality found in
fertilization, usually results in embryonic death.
Physiologically, the penetration of the cytoplasm of
an egg by more than one spermatozoa occurs in
various species including insects, reptiles, and birds,
whereas in mammals polyspermy is considered an
abnormal phenomenon that results in zygote failure
(Pepi et al., 2010). Mammals have several
mechanisms to reduce the incidence of polyspermy.
It is said that capacitation, spermatozoa transport
through several parts of the female channel (ie:
cervix, uterotubal junction), and reservoir of
spermatozoa in the oviduct regulates the number of
spermatozoa that reach the site of fertilization in
various species. In pigs, in particular, gamete
exposure to oviduct epithelial cells and oviduct
secretion can reduce the occurrence of polyspermy
(Gardner and Evans (2006). In addition to involving
the female reproductive tract, the egg itself blocks the
polyspermy to prevent fertilization by other
spermatozoa after the egg has first fertilized the egg.
Polyspermi inhibition in the egg occurs at two levels,
namely: 1) at the plasma level of the egg cell
membrane (oolemma) or vitellin and 2) on the outer
layer of the egg called the zona pellucida (ZP) in
mammals and vitelline envelope in non-mammals. At
the level of the zona pellucida, this defense is often
called a blockade zone (zone reaction) which involves
cortical exocytosis of granules after oocyte
penetration by spermatozoa, while at the oolemma
level it is called a vitellin block which involves Ca
2+
signaling (Coy and Aviles, 2010). After the
penetration of spermatozoa, cortical granules (CG)
release their contents into the perivitelline space
(perivitelline space) in an event called a cortical
reaction. The removal of CG contents changes the
property of the zona pellucida known as the zone
reaction, thereby blocking polyspermy penetration
(Pepi et al., 2010).
Oviduct Specific Glycoprotein (OVGP1) is a
glycoprotein that has been identified as a protein
secreted from unciliated secretory epithelial cells in
oviducts with a molecular weight of 65 kDa in goats
Firmawati, A., Herawati, ., Pratiwi, H., ‘Ainiyah, L. and A. S., R.
Study of Oviduct Expression Specificct Glycoprotein1 (OVGP1) on Oocyte and Goat Follicles (Capra hircus).
DOI: 10.5220/0009586500490052
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 49-52
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
49

(Pratiwi, 2017). Expression of this protein is very
dependent on estrogen levels and the oestrus phase of
the species, therefore this protein is often referred to
as estrogen-dependent glycoproteins (OGPs).
McCauley et al., (2003) also mentioned that OVGP1
expression in rats and pigs was localized in the zona
pellucida, perivitelline space, and oocyte membrane
plasma taken from the oviduct (in vivo) in the pre
embryonic period.
In the study of Coy et al., (2008) states that
OVGP1 added to cattle and pig oocytes during oocyte
maturation in vitro is known to increase blockade of
the zona pellucida and can reduce the incidence of
polyspermy so that it can increase the success of
fertilization. Seeing the potential role of OVGP1
which is very supportive in fertilization and seeing
that research on OVGP1 in goats is still rarely done,
it is necessary to conduct research on OVGP1
expression in the ovaries and OVGP1 expression in
oocytes, in order to obtain supporting data on
increasing fertilization rates in the process of in vitro
fertilization.
2 MATERIALS AND METHODS
2.1 Tools and Materials
The research material in the form of ovaries was taken
from female goat reproductive organs waste obtained
from RPH in Malang, while oocytes were obtained
from aspirations from reproductive organs waste cut
in the RPH.
2.2 OVGP1 expression on Oocytes
The immunocytochemical technique used is the
avidin-biotin-peroxidase-complex (ABC) method.
Oocytes that are included in the quality of the
Fixation on top of polylysine glass objects that have
been given paraffin and vaseline at all four ends, then
covered with a glass cover while pressing slowly.
Oocytes are fixed in a solution of ethanol: acetic acid
with a composition of 3: 1, for 2-3 days. After
fixation, oocyte preparations are taken and then
placed on a tissue to dry. Oocytes are observed under
a microscope to see oocytes. After that, the aqua-
destilation is dropped using a 1cc disposable syringe
and left for 5 minutes. Then add PBS and wait for 5
minutes. After that, it drops with hydrogen peroxidase
block for 10 minutes. Dropped PBS twice for 5
minutes (the sauce is smoked slowly with tissue).
Trypsin drops for 15 minutes and puts an incubator.
Dropped with Ultra Violet Block for 5 minutes. Then
rinsed with PBS and immediately dropped with
OVGP primary antibody antibodies for 1 hour, after
that washed with PBS twice for 5 minutes. Drop the
Biotylated link (yellow) for 30 minutes. Then
dripping PBS twice for 5 minutes. Streptavidin (Red)
drops for 30 minutes and drops with PBS for 5
minutes. Dropped with chromagen DAB for 10
minutes. And PBS drops for 5 minutes. Then in drops
of aqua-destilation for 5 minutes after that, it was
stained with methylene green and flowed with water
for 5 minutes (Schmidt, 2001).
2.3 OVGP1 Expression on Follicles
Histological preparations of ovarian organs were with
xylol I, xylol II, absolute ethanol I, absolute ethanol
II, stratified ethanol (90%, 80%, 70%, 30%), and
distilled water for 1 x 5 minutes respectively, then
washed with PBS pH 7.4 3 times. Furthermore, the
preparations were mixed in 10 mM citrate buffer pH
6 and 1 mM EDTA pH 8 for 10-20 minutes at 90 oC
then the slides were washed using distilled water. The
next stage is the process of blocking tissue peroxidase
using 3% H2O2 in methanol for 10 minutes, then
washed with PBS 3 times. The next process is
blocking the slide with 1% skim milk in PBS-tween
for 30 minutes, then the slide is washed using PBS 3
times. The next slide is given with primary antibodies
in a ratio of 1: 100 in 1% skim milk and PBS-tween.
The slides are then stored at 4oC for approximately
24 hours. Then the slide was washed with PBS 3
times. The next process is the addition of secondary
antibodies with a ratio of 1: 300 in PBS that is left for
1 hour, then the slides are washed with PBS 3 times
(Hadi, 2015).
The histological preparations were then dropped
by SA-HRP (Strep Avidin Horse Radish Peroxidase)
in a ratio of 1: 500 in PBS and incubated for 45
minutes at room temperature. Then washed again
with PBS pH 7.4 3 times, then dropped with DAB
(Diamano Benzidine) and incubated for 30 minutes at
room temperature. The slides were then washed again
with PBS pH 7.4 3 times, then counterstaining the
slides with Mayer Hematoxyler for 10 minutes. The
preparations are then washed with running water for
10 minutes, then rinsed with distilled water and dried
for about 1 night. The final stage is the mounting
process by using the glass and then covered with a
glass cover. Subsequent results were observed using
a microscope with a magnification of 400x (Hadi,
2015).
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
50
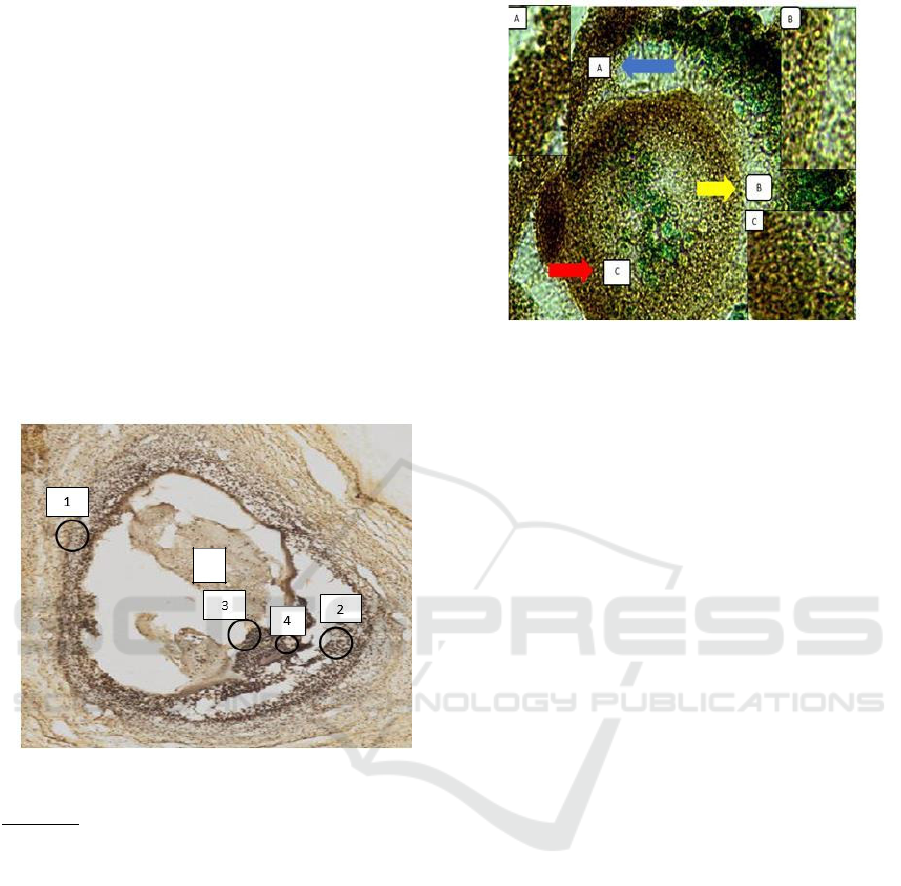
3 RESULT AND DISCUSSION
3.1 OVGP1 Expression in Goat
Follicles
McCauley et al., (2003) mention Epihelliacell
OVGP1 Glycoprotein (EOGPs) in mice and pigs
localized in the zona pellucida, perivitelline space,
and plasma oocyte membranes taken from oviducts
(in vivo) and embryos. In this study, based on
immunohistochemical analysis shows that the
expression of OVGP1 (Oviduct Specific
Glycoprotein 1) in goat follicles is found in the
external theca cells, internal theca, liquor follicular,
granulosa, and zona pellucida. The results of OVGP1
expression in goat follicles can be seen in Figure 1
below.
Figure 1. OVGP1 expression in Goat Follicles
Information:
1. Theca external cells
2. Internal theca cells
3. Granulosa cells
4. Zona Pelusida
5. Liquor follicular
3.2 OVGP1 Expression on Goat
Oocytes
Based on the immunocytochemical analysis, OVGP1
expression on oocytes shows that there are cumulus
oophorus complexes, zona pellucida, and
perivitelline space. localized in part of the zona
pellucida, perivitelline space, and plasma oocyte
membrane is taken from the oviduct (in vivo) and the
embryo. The results of OVGP1 expression in goat
oocyte can be seen in Figure 2 below.
Figure 2. OVGP1 expression on Goat Oocytes
Information:
a) Cumulus oophorus Complex
b) Pelusida Zone
c) Perivitelin space
The OVGP1 expression of the results of this
study is in the internal and external parts of the theca.
OVGP1 which can be assumed also plays a role in the
process of follicular maturation along with GDF-9
which results from the maturation process of this
follicle that will produce estrogen synthesis. On the
part of granulosa cells that are expressed in follicles
and oocytes, this can play a role in the nourishment
process of eggs during follicle ripening and
maturation of oocytes from goats, whereas OVGP1
which is expressed in the zone of the pellucid and
perivitelline space shows that OVGP1 will bind to
TGF- β to inhibit polyspremia in the process of
fertilization. In addition, OVGP1 together with GDF-
9 late has been shown to play a role in the process of
folliculogenesis and oocyte maturation. Based on the
results of this study, it can be concluded that OVGP1
also plays a role in the process of follicular and oocyte
maturation along with GDF-9 and TGF-β as well as
zone and vitellin blockade. In addition to the role of
growth factors that directly interact with OVGP1 as a
receptor in the signaling process, there are also
important roles of the FSH, LH and estrogen
hormones that play an important role together with
OVGP1. Where the increase in OVGP1 levels in the
follicular phase is higher than in OVGP1 levels in the
luteal phase. It can be concluded that OVGP1 in
addition to playing a role in the process of
fertilization, polyspermia blockade, early embryonic
development, OVGP1 also plays an important role in
the process of preovulation, ovulation, and oocyte
maturation.
Study of Oviduct Expression Specificct Glycoprotein1 (OVGP1) on Oocyte and Goat Follicles (Capra hircus)
51

According to Buhi (2002), OVGP1 expression
depends on the stage of the estrous cycle and is
associated with circulating estrogen concentrations.
Estrogen will stimulate OVGP1 expression. The
synthesis of estrogen occurs from aromatization
enzymes that produce androgen hormones whic are
converted to estradiol
17β under the influence of
FSH (Liben, 2000). In the ovarian follicle, the
hormone estrogen will be formed by granulosa cells
through enzymatic reactions. The activity of these
granulosa cells is related to the activity of the
hormones FSH and estrogen. OVGP1 can indirectly
increase some of the effects of the FSH related to the
activity of GDF-9 aromatization in granulosa cells
and the promotion of DNA synthesis in follicular
cells (Laheri et al., 2017). OVGP1 expression is
influenced by hormonal levels while still in the ovary,
so that OVGP1 expression is related to hormonal
influences (Buhi, 2002). OVGP1 expression in the
zona pellucida indicates that OVGP1 will later bind
to TGF-β which is TGF- this is a receptor of OVGP1
which will then cause complex bonding so as to
increase defense of the zone and vitellin blockade so
as to prevent polyspermia.
4 CONCLUSIONS
OVGP1 expression in goat follicles is expressed in
the external theca cells, internal, granulosa, zona
pellucida and liquor follicle while OVGP1 expression
in oocytes is expressed in the cumulus oophorus
complex, the zona pellucida, and perivitelline space.
ACKNOWLEDGMENTS
We would like to thank the Faculty of Veterinary
Medicine, Universitas Brawijaya for providing
research assistance and support funds until the
completion of the research.
REFERENCES
Albert, B., Johnson, A., Lewis, J., Raff, M., Roberts, K. &
Walte, P. (2008). Molecular Biology of the Cell. 7th ed.
Garland Science. USA. 1269-1304.
Anghel, A. H., Zamfirescu, S., & Coprean, D. (2009).
Influence of Vitamin E on Microscopic and Oxidative
Parameters of Frozen-Thawed Ram Semen. Lucrari
Stiintifice 54: 261-265.
Anguilar, J. & Reyley, M. (2005). The uterine tubal fluid:
secretion, composition and biological effects. Animal
Reproduction 2: 91-105
Buhi, W.C. (2002). Characterization and Biological Roles
of Oviduct-specific, Oestrogen-dependent
Glycoprotein. Reproduction 123: 355-362.
Buhi, W.C., Alvarez, L.M., Choi, I., Cleaver, B.D., &
Simmen F.A. (1996). Molecular Cloning and
Characterization of an Estrogen-Dependent Porcine
Oviductal Secretory Glycoprotein. Biology of
Reproduction 55: 1305-1314.
Coy P. & Aviles, M. (2010). What Controls Polispermi in
Mammals, the Oviduct or the Oocyte?. Biological
Reviews 85: 593–605.
Papi, M., Brunelli, R., Sylla, L., Parasassi, T., Monaci, M.,
Maulucci, G., Missori, M., Arcovito, G., Ursini, F. &
Spirito, M.D. (2010). Mechanical Properties of Zona
Pellucida Hardening. Eur Biophys J 39:987–992.
Parrish, J. J., Susko-Parrish, J.L., Handrow, R.R., Sims,
M.M. & First, N.L (1989). Capacitation of Bovine
Spermatozoa by Oviduct Fluid. Biology Of
Reproduction 40: 1020-1025.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
52
