
Expression of Estrogen Beta (Ers Β) Receptor and Ovarian
Histopathology Changes in Rats (Rattus Norvegicus) Ovarian
Hypofunction Model
Aulia Firmawati
1
, Mitra Artha Kurnia Hutabarat
2
and Herlina Pratiwi
3*
1
Reproduction Laboratory, Faculty of Veterinary Medicine, Brawijaya University, Indonesia
2
Undergraduate Students of the Faculty of Veterinary Medicine, Brawijaya University, Indonesia
3
Embryology Laboratory Faculty of Veterinary Medicine, Brawijaya University, Indonesia
Keywords: ERs β, Folliculogenesis, GnRH antagonists, Ovarian hypofunction, Rats
Abstract: One of the reproductive disorders that are often found in breeders in Indonesia is ovarian hypofunction.
Ovarian hypofunction is a pathological condition in the ovary that is characterized by a decrease in ovarian
function that causes inhibition of folliculogenesis and failure of ovulation. The purpose of this study was to
develop ovarian hypofunction animal models through cetrorelix acetate induction and observe their effects on
the expression of estrogen beta receptors (Ersβ) and histopathological changes in the ovaries. This study used
three groups of female Wistar strains (Rattus norvegicus), with ages 8-10 weeks, and body weight 150-180
grams. The treatments in this study included a control group (KN) without cetrorelix acetate, the first group
(P1) was treatment group with an injection of cetrorelix acetate 0.009 mg/kg BW, and the second group (P2)
was treatment group with an injection of cetrorelix acetate exposure 0.0135 mg/kg BW. Expression of beta
estrogen receptors (Ersβ) in the ovaries was analyzed by immunohistochemical methods, and the data were
analyzed using the BNJ test (p <0.05). The ovarian histopathological changes were analyzed by the
hematoxylin-eosin (HE) staining method, then analyzed qualitatively. The results of this study indicate that
the treatment groups P1 and P2 with GnRH antagonists differ significantly compared to the negative control
group. The P2 treatment group had the highest reduction in estrogen receptor expression with Ers β by 92.2%.
The result of histopathological in P1 and P2 treatment groups were able to inhibit the development of antral
follicles. The conclusion of this study is that cetrorelix acetate as GnRH antagonists can reduce estrogen
receptor expression and inhibit folliculogenesis in ovarian histopathology.
1 INTRODUCTION
The highest incidence of reproductive disorders in
community farms in Indonesia is ovarian
hypofunction. The incidence rate of ovarian
hypofunction in East Java in 2010-2017 was 9.28%
(Hermadi, 2015), while ovarian hypofunction in
Rembang Regency, Central Java, was 6.25%
(Sutiyono et al., (2017) and in the Regency Enrekang,
South Sulawesi Province at 71% (Yahya, 2017) and
in Jambi province it was reported that the incidence
of ovarian hypofunction had the second- highest
percentage of events at 19.32% (Rosadi et al., 2018).
Ovarian hypofunction is a pathological condition
characterized by a decrease in ovarian activity is
showing signs of lust and producing ovum. Ovum
produced under conditions of ovarian hypofunction
generally has low fertility, making it difficult and
even cannot be fertilized despite good quality
spermatozoa. Ovarian hypofunction is the most
frequent reproductive disorder due to
mismanagement of feed and reproductive hormone
mechanisms (Pradhan and Nakagoshi, 2008).
According to Gitonga, (2010) Cows that experience
ovarian hypofunction are often found with the
occurrence of silent heat, sub estrus (lust without
ovulation), irregular lust cycle and the onset of
postpartum lust. Ovarian hypofunction that is not
treated immediately can cause ovarian atrophy that is
irreversible.
In conditions of ovarian hypofunction Ovarian
histopathological features can show ongoing
folliculogenesis in the ovary. Ovarian follicles based
on their development are divided into several levels
including primordial follicles, primary follicles,
Firmawati, A., Hutabarat, M. and Pratiwi, H.
Expression of Estrogen Beta (ERs ) Receptor and Ovarian Histopathology Changes in Rats (Rattus norvegicus) Ovarian Hypofunction Model.
DOI: 10.5220/0009586400410048
In Proceedings of the 6th International Conference on Advanced Molecular Bioscience and Biomedical Engineering (ICAMBBE 2019) - Bio-Prospecting Natural Biological Compounds for
Seeds Vaccine and Drug Discovery, pages 41-48
ISBN: 978-989-758-483-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
41

growing follicles, and de Graaf follicles. Disrupted
folliculogenesis can eliminate follicles in various
stages and then develop into follicular atresia.
Ovarian hypofunction will show a delay in the
development of primary follicles, secondary follicles,
and the presence of atresia follicles in quite high
numbers. Folliculogenesis that occurs is difficult to
reach the ovulation stage so that no tertiary follicles,
de Graaf follicles or corpus luteum are found
(Hestianah et al., 2014).
The production of the hormone estrogen
continues to increase when de Graaf follicles will
ovulate. The effect of the hormone estrogen in tissue
is closely related to the activation of the estrogen
receptor that the tissue has. Obstructed ovulation will
certainly affect the production of the hormone
estrogen followed by activation of its receptors. The
production of the hormone estrogen can decrease
because follicles do not develop thereby reducing
estrogen receptor activation in some target tissues.
Estrogen can diffuse into ovarian, uterine, and
mammary glands systemically (Caldon, 2014).
Ovarian hypofunction can be caused by the
disruption of hormonal regulatory mechanisms by
suppressing reproductive hormone synthesis. One of
the causes of disruption of the reproductive hormone
mechanism is the use of GnRH antagonists namely
Cetrorelix acetate in humans which aims to suppress
ovum production so that it can cause a decrease in
ovarian function or ovarian hypofunction. GnRH
antagonists are indicated to suppress the luteinizing
hormone surge (LH) that is too early in women. LH
suppression occurs due to a decrease in the number of
activated GnRH receptors on gonadotropin cells
(Beckers and Reila, 1997).
2 MATERIALS AND METHODS
2.1 Tools and Materials
The tools used in this study include therumo® 1 cc
syringes, terumo® 3 cc syringes, blades, surgical
scissors, anatomical tweezers, serological tweezers,
surgical boards, petri dishes, and pins, microtomes,
incubators, and optilab microscopes.
Materials used in this study include feed in the
form of rabbit pellets (SP®), husks, and sufficient
water, Phosphate Buffer Saline (PBS), formalin,
alcohol, xylol, 0.9% physiological NaCl, paraffin,
Hematoxillin-Eosin dye, and entellan, PBS, primary
antibody ERS β brand abcam® (ab288), secondary
antibody labeled peroxidase, normal Horse serum
2.5% brand abcam® (ab7484), hydrogen peroxide,
methanol and chromogen diaminobenzidine
tetrahydrochloride (DAB) brand abcam® (ab64238).
2.2 Preparation of Animal Ovarian
Hypofunction Models
Rats were acclimatized for 7 days to be able to adapt
to the new environment. Rats are given feed in the
form of rabbit pellets (SP®) and the provision of
drinking water by adlibitum. Rats were divided into 3
treatment groups including: control group (KN), the
untreated group was only given a placebo NaCl for 17
days. The treatment group was a cetrorelix acetate
dose of 0.009 mg/kg BW given for 17 days (P1). The
third group was the treatment group with a dose of
cetrorelix acetate of 0.0135 mg/kg BW.
2.3 Vaginal Swab Preparations
The method of making vaginal swab preparations
carried out in this study by immersing cotton bud in
physiological NaCl then the mice to be swab vagina
will be placed in a dorsal lying position, after which
a vaginal swab is carried out by inserting a cotton bud
soaked physiologically NaCl in the vagina by rotating
360
o
. Then, the cotton bud is removed on the slide
and allowed to dry and then fixed using alcohol. The
preparations that have been fixed with alcohol and
which have dried then are stained with Eosin
Negrosin for 15 minutes, then rinsed with running
water with a small flow of water and rinsed slowly.
After that, the results of vaginal swabs are observed
under a microscope with a magnification of 100x and
400x to see vaginal cells. In this study, a vaginal swab
was carried out before interfering with the GnRH
antagonist to equalize the estrous cycle of rats before
starting treatment in this study.
2.4 Collect of Ovary Organs
The rats were euthanized by cervical dislocation.
Dislocation is a condition where the joints completely
change position without contact with each other
(Manuabada and Putu, 2017). Neck dislocation is a
physical euthanasia technique by separating the joints
between the skull and brain from the spinal cord in
the vertebrae. This technique is done by placing the
mouse in a ventral fall position, the nape is held with
tweezers, then pulled at the base of the tail and the
whole body until the mouse dies (Isbagio, 1992).
The rats that have died are dissected vertically
from the posterior abdomen to the anterior region and
then opening the abdominal and chest cavity. The
ovaries were taken together with the uterus, then
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
42

separate the ovaries from the mesovarium. The
ovaries are prepared from the surrounding fat and
washed with physiological NaCl.
2.5 Preparation of Ovarian
Hhistopathology
The histological preparations consist of fixation,
dehydration, clarification, paraffin infiltration,
embedding, sectioning, sticking to glass objects and
coloring. Histopathological changes that will be
observed in the description of ovarian histopathology
are folliculogenesis, histopathological changes
include necrosis, inflammation or other cell damage.
2.6 Observation of Ovarian Estrogen
Receptor Beta (ERsβ) Expression
Observation of the expression of estrogen beta
receptors (ERs β) was observed using the
immunohistochemical method. Ovarian
histopathological preparations without coloring are
incubated for > 24 hours to facilitate
deparaffinization. The preparations are soaked in
xylol 3 times for deparaffinization, removing paraffin
from the tissue as much as possible. The preparations
are then soaked in stratified alcohol in sequence
(95%, 90%,80%, 70%) for rehydration, ie re-entering
the liquid into the tissue making it easier to stain, then
stored in the refrigerator 30
0
C for > 24 hours. The
preparations are washed in distilled water 3 times
each for 3 minutes. The preparations are immersed in
3% hydrogen peroxide mixed with methanol in a
room temperature humidity chamber for 40 minutes,
then washed in PBS for over 3 x 3 minutes. The
preparations were incubated with 50% normal serum
horse as much as 50 µL for one night in a humidity
chamber at 3oC and rinsed again with PBS for 3 x 3
minutes.
Preparations added 40 µL (primary antibody)
anti-ERs β brand abcam® (ab288), for one night at
3oC, then rinsed again with PBS for 3 x 5 minutes.
The preparations are added with abcam® brand anti-
receptor secondary antibodies (ab7484), 40 µL for 60
minutes at room temperature, then washed with PBS
for 3 x 3 minutes. The preparation is dripped with one
drop peroxidase, left for 40 minutes, then washed
with PBS for 3 x 3 minutes.
The preparation is rinsed again with distilled
water. Preparations were added chromogen DAB
(3,3-diaminobenzidine tetrahydrochloride) abcam®
(ab64238) as much as 40 µL for 20 minutes at room
temperature then washed in distilled water 3 x 5
minutes and countered with hematoxylin for 1 minute
at room temperature. The preparation was washed
again in the water at pH 8, 3 x 1 minute and let it dry,
mounting using an entelan, then the preparation was
observed with an optical microscope.
ERs β in the ovary are observed by calculating the
average number of cells expressing β ERs.
Observation of β ERs in the ovary can be observed in
theca cells and granulosa cells. ERs β calculation is
done at 400x magnification with 5 visual fields per
slide and then analyzed with the help of Immunoratio
software (Ridwan et al., 2015).
3 RESULTS AND DISCUSSION
Effect of GnRH Antagonists on Estrogen Beta
Receptor Expression (ERsβ) in the Ovary Expression
of beta estrogen receptors (ERsβ) in the ovary can be
observed by immunohistochemical (CPI) methods.
Ovarian cells that express β ERs will be colored easily
brown to dark brown with a certain area. Expression
of beta estrogen receptors (ERs β) in the ovary will be
expressed in the cells of granulosa cells and germinal
epithelium. Physiologically, ERs β show more
expression than α ERs in the ovary (Wang et al.,
2000).
In this study, the expression of estrogen receptors
is expressed in the theca cells of ovarian follicles. The
estrogen receptor's expression is colored light brown
to dark brown, this indicates that the theca cells
express the presence of estrogen that binds to the
estrogen receptor in the theca cell. According to Cui
et al., (2013) theca cells are cells that synthesize the
formation of estrogen in cooperation with granulosa
cells during the process of folliculogenesis and
ovarian follicle maturation. Estrogen produced will
stimulate proliferation and differentiation of theca
cells and granulosa cells in ovarian follicles in the
process of ovarian follicle maturation. Theca cell
differentiation is influenced by several factors, one of
which is the Bone Morphogenic Proteins (BMP) and
Growth Differentiation Factor-9 (GDF-9) expressed
by estrogen-estrogen receptor binding in the
transcription process (Young et al., 2010).
The average calculation result of ERs β expression
obtained in the control group was 62.58 ± 1.4, the P1
group was 33.80 ± 1.6, and the P2 group was 4.87 ± 1.6.
ERs expression of β in the P1 and P2 groups decreased
compared to the control group.
Data obtained from the expression of ERs β showed a
greater control group (62.58 ± 1.4) when compared to
the P1 group (33.80 ± 1.6) and P2 group (4.87 ± 1.6).
The ERs β expression values can be seen in table 1.
Expression of Estrogen Beta (ERs ) Receptor and Ovarian Histopathology Changes in Rats (Rattus norvegicus) Ovarian Hypofunction
Model
43

Table 1. Effects of GnRH antagonists on the expression of
ERsβ in rats ovarian hypofunction
Group
Average Expression of ERs β
±
standard of deviation
KN (placebo)
62,58 ± 1.4
c
P1 (cetrorelix acetat
33,80 ± 1.6
b
0.009 mg/Kg BW)
P2 (cetrorelix acetat
4,87 ± 1.6
a
0.0135 mg/Kg BW)
Information :
The notation a, b, c shows that there is a significant difference
between one treatment and another
Based on the table above shows that the higher the
dose of GnRH antagonists, the lower the expression
of ERs β in rat ovaries, the decrease in estrogen
receptor beta receptor is due to the response of GnRH
antagonists, which gives inhibition of anterior
pituitary secretion to secrete primer reproductive
hormones. Cetrorelix acetate as one of the GnRH
antagonists competes with GnRH in the body to bind
to membrane receptors on pituitary cells and inhibits
the release of Follicle Stimulating Hormone (FSH)
and Luteinizing Hormone (LH), thereby delaying LH
surge, and it will result in inhibition of ovulatory
processes (Rodney, FSH) and Luteinizing Hormone
(LH).
The existence of obstacles in these pituitary cells
causes obstacles in the process of folliculogenesis.
Hampered the process of eating folliculogenesis will
result in a decrease in estrogen production which will
then cause a decrease in the binding of estrogen
hormones to estrogen receptors, one of which is ERs
Beta. With this decreased hormone-receptor binding,
the expression of beta ERs becomes inactive and
unexpressed, this is in accordance with the results in
this study that decreased expression of beta receptors
in rat ovaries treated with GnRH antagonists. In this
case, the administration of cetrorelix acetate affects
the decrease in expression of beta estrogen receptors
in the ovary. The β ERs expression in ovaries can be
seen in (Figure 1.)
In this study, it was shown that the decrease in the
ERs expression of the control group was greater than
the P1 and P2 groups. Decreased expression of ERs β
in the treatment groups P1 and P2 are caused by
GnRH antagonistic intervention. Exogenous GnRH
antagonists that are competitive with endogenous
GnRH in binding to estrogen receptors will inhibit
endogenous GnRH to inhibit FSH and LH secretion
in the anterior pituitary. This inhibition of FSH and
LH causes a decrease in the level of FSH and LH in
the blood. The effect of FSH secretion inhibition
inhibits the process of folliculogenesis and inhibits
ovulation due to low levels of LH in the blood. So that
this causes estrogen which is synthesized during the
formation, development, maturation of follicles until
ovulation occurs. It will experience a disruption in
secreting the hormone estrogen in the blood
circulation so that it experiences inhibition of
folliculogenesis and inhibition of ovulation.
Figure 1. Expression of β ERs by the Imunnohistochemical
method in the ovary (400x) Information:
1: Control (placebo)
2: Group P1 (Cetrorelix acetate 0.009 mg / kg BW)
3: Group P2 (Cetrorelix acetate 0.0135 mg / Kg BW)
: Estrogen Receptor β
In addition, a decrease in estrogen production will
cause the lack of estrogen hormone binding with
estrogen receptors both ERs β and ERs that is formed
so that gene expression also decreases and can cause
no emergence of estrous performance. ERs β work is
known to affect gamete transport, development, cell
growth in the reproductions channel with the presence
of GnRH inhibition, which can cause gamete
transport disorders, one of which is the disruption of
the process of folliculogenesis, LH surge disruption
which causes obstruction of ovulation, cell growth
and the growth of the female reproductive tract.
3.1 Effects of GnRH Antagonists on
Changes in Ovarian Histopathology
In this study, we did a vaginal swab first to determine
the last reproduction phase before the euthanized.
And based on the results of a vaginal swab conducted
before euthanasia showed experimental animals in
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
44

the control group (KN), group P1, and group P2 was
in the metestrus phase. Physiologically in the
metestrus phase is characterized by the development
of de Graaf follicles that have ovulated to the corpus
luteum. The process of maturation of the corpus
luteum produces progesterone whose concentration
will peak on the 6th day after ovulation. Progesterone
plays a role in influencing the reproductive tract to
maintain pregnancy in the event of fertilization
(Akbar, 2010).
The control group showed a follicle that had just
ovulated which was called the corpus haemoragicum
which would develop into the corpus luteum (Figure
2A). Follicles that successfully reach ovulation are
dominant follicles that have gone through 2 to 3
follicular waves during the estrous cycle period.
Dominant follicles produce estrogen which can
suppress the growth of other small follicles
(Jainudeen and Hafez, 2000). Besides copus
haemoragicum, corpus luteum and primary,
secondary and atresia follicles are also found. This
shows that the control group that was only given a
placebo still showed normal ovarian activity.
A follicular wave is defined as a process of
follicular growth that is synchronous with several
small follicles. One of these small follicles is selected
to grow into a dominant follicle, while the other small
follicles will stall their growth into follicular atresia.
Atresia follicles that are formed will cause a new
follicular wave that is the second follicular wave. The
dominant follicle in the second follicular wave will
become anovulatory, while the dominant follicle in
the third wave will ovulate (Siregar, 2010). Other
follicles such as primary follicles and secondary
follicles encountered in the negative control group
indicate a new wave of follicles in the ovaries.
P1 group by giving Cetrorelix acetate dose 0.009
mg/kg BW showed that there was a small number of
tertiary follicles observed and the formation of atresia
follicles was like the control group. This can be
caused when the animal is given induction of GnRH
antagonist ovarian status in the mouse model of
ovarian hypofunction cell death occurs in the
development of follicles de graf into corpus luteum.
Physiologically, de graf follicles that have failed in
ovulation then the de graf follicles will be eliminated
and turn into follicular atresia. Whereas in the P2
group that was given a Cetrorelix acetate dose of
0.0135 mg/kg BW the primary and secondary
follicles were observed in large amounts compared to
follicular development. The growth of primary and
secondary follicles classified as quite a lot in the P2
group compared to the P1 and K groups can be
assumed that in the group given the GnRH antagonist
intervention experienced inhibition of
folliculogenesis so that the primary and secondary
follicles do not experience follicular development
into the antral follicle. In addition, the P3 group also
found a lot of follicles that experience necrosis.
Necrosis is the first step of ovarian cell damage
that can result in apoptosis (cell death) so that it will
decrease the secretion of the hormone estrogen and
decrease the expression of ERs. An image of the
changes in ovarian histopathology in mice with
ovarian hypofunction models can be seen in Figure 2.
GnRH antagonists induced in the treatment group
gave a difference between the treatment group and the
negative control group. The increasing number of
primary and secondary follicles in the negative
control group, the P1 treatment group, and the P2
treatment group occurs because the growth of primary
and secondary follicles does not depend on GnRH so
that the induction of GnRH antagonists has no effect
on the preantral follicle (Puspitasari, 2011). The
development of primordial follicles into primary
follicles and primary follicles into secondary follicles
depends on the nutrition provided (Ramadhani et al.,
2017). The decrease in the number of tertiary follicles
in groups P1 and P2 shows that GnRH antagonists are
able to inhibit the development of antral follicles that
depend on GnRH. The increase in the number of
atresia follicles in the antral follicle along with the
increasing dose of GnRH antagonist shows that the
induction of GnRH antagonist is able to increase the
number of atresia follicles.
Expression of Estrogen Beta (ERs ) Receptor and Ovarian Histopathology Changes in Rats (Rattus norvegicus) Ovarian Hypofunction
Model
45
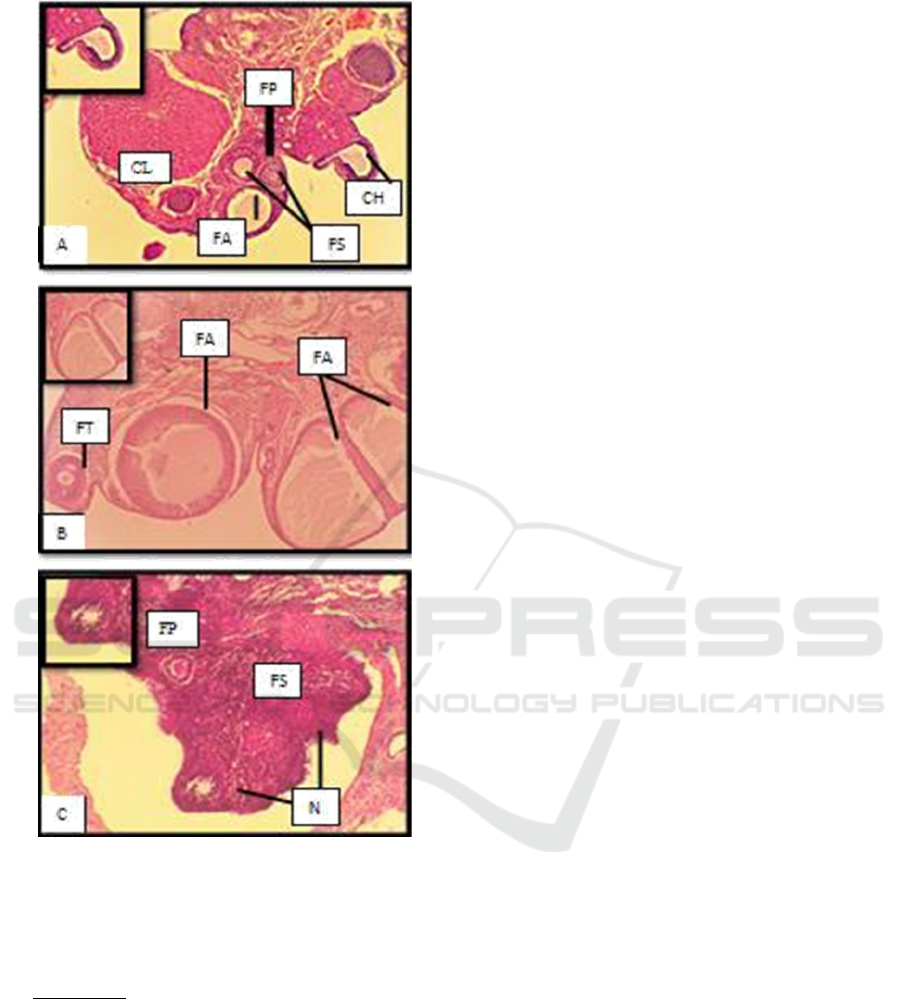
Figure 2. Histological features of folliculogenesis in the
ovaries of Rats (HE, 40x); FA (Atretic follicular); FP
(Primary follicles); FS (Secondary follicles); FT (Tertiary
Follicles); FD (De Graaf Follicular); CH (Corpus
Haemoragicum); N (Necrosis)
Description :
(A) K : Control (placebo)
(B) P1: Cetrorelix acetate (0.009 mg / Kg BW)
(C) P2 : Cetrorelix acetate( 0.0135 mg / Kg BW)
Follicles that undergo a degenerative process
before reaching the ovulation stage are called
follicular atresia. This degenerative process occurs
normally to select follicles, so only follicles that
contain healthy oocytes are ovulated. According to
Hsueh et al., (1994) there are 3 theories that explain
the causes of atresia in a follicle. The first theory
explains that follicles experience atresia due to
deficiencies in the genetic component, oocyte
cytoplasm, somatic cells, and follicular environment.
The second theory explains that the follicle is exposed
to teratogenic factors that carry the follicle into the
degenerative pathway. The third theory explains that
atresia is the fate of all follicles when going through a
critical stage in the follicle unless the critical stage
coincides with FSH stimulation, the follicle will
continue to develop and become a dominant follicle.
An increase in the number of follicular atresia with
increasing dose of GnRH antagonist shows decreased
FSH stimulation due to induction of GnRH antagonist
and does not coincide with the critical stage of the
follicle. Durlinger et al., (2000) research on atresia
follicles in pre-ovulatory follicles due to GnRH
antagonist states that evaluation of atresia follicles is
done by observing the thickness of the granulosa cell
layer which is getting thinner. Cumulus oophorus in
de Graaf follicles also experiences thinning. Atresia
follicles in the growing follicles and de Graaf follicles
will cause the following changes: (1) The oocyte
becomes the first degenerated structure then
disappears; (2) The zona pellucida swells and
disappears most recently; (3) The granulosa cells
degenerate so that the granulosa membrane
boundaries become irregular because the cells are
scattered and disappear as well; (4) Internal theca cells
in the follicle will become theca lutein cells,
eventually the atresia follicles will become the corpus
albikan (Hestianah et al., 2014).
Further research on the use of GnRH antagonists
in ovarian stimulation for IVF programs is ongoing.
According to Macklon and Fauser, (2000) the initial
step of the IVF program, namely the administration of
GnRH antagonists, aims to utilize dominant follicles
that develop from primordial follicles to secondary
(preantral) follicles. Dominant follicles are follicles
with granulosa cells which are more sensitive to FSH
stimulation. This causes the less FSH to be stimulated,
the greater the potential for FSH to only affect the
dominant follicle. Non-dominant follicles will lack
FSH then become follicular atresia. A decreased FSH
concentration will leave a dominant follicle ready for
maturation. GnRH antagonists were given at a dose of
0.0135 mg/kg BW in the P2 group causing damage in
the form of necrosis of the antral follicle in the ovary.
This is in accordance with research Safitri et al.,
(2012) regarding the condition of ovarian
hypofunction caused by malnutrition showing
histopathological conditions in the form of necrosis,
congestion, and edema in ovarian follicles.
Degeneration and necrosis of ovarian follicles indicate
that there is no development in ovarian follicles. This
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
46

is in accordance with the case study of Kesler and
Gaverick, (1982) which states the condition of ovarian
hypofunction is caused by hormonal deficiency and
imbalance resulting in anestrus and estrus which is not
accompanied by ovulation. This hormonal imbalance
affects the function of the anterior pituitary so that the
production of FSH and LH is low which causes the
ovaries to not develop.
According to Griesinger et al., (2005) GnRH
antagonists were able to reduce FSH levels by 75%,
LH levels by 84%, and estrogen until undetectable
within 36 hours. The activity of the hormone estrogen
requires binding to receptors to stimulate stromal and
epithelium cell proliferation. and β receptors work
homologously with each other and have a high affinity
for estrogen. Decreased estrogen levels due to the
administration of GnRH antagonists in the treatment
groups P1 and P2 will reduce estrogen binding
estrogen receptors thereby reducing the expression of
estrogen receptors in both ERs β and α. This causes
the transcription process that occurs because estrogen
bonds and estrogen receptors decrease and cause gene
expression to also decrease so that it interferes with
cell proliferation in the target cell. The proliferation of
disturbing cells will cause inhibition of
folliculogenesis in the ovary (Cooke et al., 1998). This
inhibition of folliculogenesis will result in decreased
estrogen levels. A decrease in estrogen levels and
estrogen bind with ERs causes no positive feedback to
the hypothalamus so that GnRH does not stimulate the
anterior pituitary to produce LH, and LH surge does
not occur so ovulation will never occur (Hafizuddin et
al., 2012).
4 CONCLUSIONS
GnRH antagonists were able to reduce ERsβ
expression in white rats in the P2 treatment group at
a dose of 0.0135 mg / Kg BW and GnRH antagonists
were able to inhibit the development of ovarian
follicles namely tertiary follicles and de Graaf
follicles in the ovarian histopathology picture in the
P1 and P2 treatment groups and cause the occurrence
of necrosis in the ovarian histopathology picture of
the P2 treatment group approaching the condition of
ovarian hypofunction.
ACKNOWLEDGMENTS
We would like to thank to LPPM Universitas
Brawijaya and the Faculty of Veterinary Medicine,
Universitas Brawijaya for providing research
assistance and support funds until the completion of
the Ovarian Hypofunction Rats Model with the
antagonist GnRH Induction.
REFERENCES
Beckers, T., Reila¨nder, H. & Hilgard P. (1997).
Characterization of Gonadotropin-releasing Hormone
Analogs Based on a Sensitive Cellular Luciferase
Reporter Gene Assay. Anal Biochem; 251:17–23.
Caldon, C. E. (2014). Estrogen Signaling and The DNA
Damage Respones in Hormone Dependent Breast
Cancers. Frontiers in Oncology, 4 (106), 1-6.
Cooke, P. S., Buchanan, D. L., Lubahn, D. B. & Cruncha,
G. R. (1998). Mechanism of Oestrogen Action:
Lessonfrom the Oestrogen Receptor-Biol. Reprod 59:
470-475.Cui, J., Yong S., Rena L. 2013. Estrogen
Synthesis and Signaling Pathways During Ageing:
From Periphery to Brain. Trends Mol Med
19(3):197-209.
Durlinger, A. L. L., Piet K., Bas K., Anton J. G., Jan T. J.
U., & Axel P. N. T. (2000). Apoptotic and Proliferative
Changes During Induced Atresia of Pre-ovulatory
Follicles in Rat. Human Reproduction Vol. 15(12):
2504-2511.
Edson, M. A., Ankur K. N., & Martin M. M. (2009). The
Mammalian Ovary from Genesis to Revelation.
Endocrine Reviews Vol. 30(6): 624-712.
Gitonga, P. N. (2010). Postpartum Reproductive
Performance of Dairy Cows in Medium and Large
Scale Farms in Kiambu and Nakuku Districts of Kenya.
University of Nairobi Faculty of Veterinary Medicine.
Griesinger, G., Ricardo F., & Klaus D. (2005). GnRH
Antagonist in Reproductive Medicine. Arch Gynecol
Obstet Vol. 273: 71-78.
Hafizuddin, Tongku, N. S., & Muslim A. (2012). Hormon
dan Perannya dalam Folikuler pada Hewan Domestik.
JESBIO Vol. 1(1): 21-24.
Hermadi, H. A. (2015). Pidato. Pemberantasan Kasus
Kemajiran pada Ternak Menuju
Kemandirian di Bidang Kesehatan Reproduksi Hewan dan
Ketahanan Pangan di Indonesia. Surabaya: Universitas
Airlangga.
Hestianah, E. P., Chairul, A., Suryo, K., & Lita, R. Y.
(2014). Buku Ajar Histologi Veteriner Jilid 2.
Surabaya: Universitas Airlangga Press.
Hsueh, A. J. W., Hakan, B., & Alex, T. (1994). Ovarian
Follicle Atresia: A Hormonally Controlled Apoptotic
Process. Endocrine Reviews Vol. 15(6): 707-724.
Isbagio, D. W. (1992). Euthanasia pada Hewan Percobaan.
Media Litbangkes Vol. 2 (1): 18-24.
Jainudeen, M.R. & Hafez, E.S.E. (2000). Cattle and
Buffalo. In B. Hafez, and E.S.E. Hafez (Eds.).
Reproduction in Farm Animals.
Lippincott Williams and Wilkins, Philadelphia. Halaman:
159-171.
Expression of Estrogen Beta (ERs ) Receptor and Ovarian Histopathology Changes in Rats (Rattus norvegicus) Ovarian Hypofunction
Model
47

Kesler, D.J. & Garverick, H.A. (1982). Ovarian Cysts in
Dairy Cattle : A review. Journal of Animal Science 55:
11471159.
Lestari, C.M.S., Purbowati, E., Dartosukarno, S., Rianto, E.
(2014). Sistem Produksi dan Produktivitas Sapi Jawa-
Brebes dengan Pemeliharaan Tradisional. (Studi Kasus
diKelompok Tani Ternak Cikoneng Sejahtera dan
Lembu Lestari Kecamatan Bandarharjo Kabupaten
Brebes). J. Peternakan Indonesia 16(1): 8- 14.
Li, H. & Ri-Cheng, C. (2017). Follicular Development and
Oocyte Growth. Shanghai: Springer International
Publishing.
Manuaba, I. B. T. W dan Putu A. 2017. Buku Panduan
Belajar Koas: Ilmu Bedah. Denpasar: Rumah Sakit
Umum Pusat Sanglah.
Pradhan, R. & Nakagoshi, N. (2008). Reproductive
Disorders in Cattle Doe to Nutritional Status. J of
Inter Dev and Coop 14: 45-66.
Puspitadewi, S. & Sunarno. Potensi Agensia Anti Fertilitas
Biji Tanaman Jarak (Jatropha curcas) dalam
Mempengaruhi Profil Uterus Mencit (Mus musculus)
Swiss Webster. Artikel Penelitian Vol.5(2): 55-60.
Puspitasari, Y. (2011). Efek Ekstrak Etanol Biji Pepaya
(Carica papaya Linn) terhadap Kadar 17-Β Estradiol
dan Folikulogenesis pada Mencit Betina (Mus
musculus) [THESIS]. Fakultas Kedokteran. Universitas
Airlangga.
Ramadhani, S. A., Iman, S., Ni, W. K. K., & Adi W. (2017).
Pengendalian Folikulogenesis Ovarium dengan
Pemberian Ekstrak Biji Kapas. Jurnal Sain Veteriner
Vol. 35(1): 71- 80.
Ridwan, A. J., Sri, S., & Abdul, H. H., & Bethy S. H.
(2015). Analysis of Immunoexpression of Estrogen
Receptor Beta and Extracellular Matrix
Metalloproteinase Inducer (EMMPRIN) on Testicular
Seminomas Nonrecurrence and Recurrence. Journal of
Medicine and Health Vol. 1(2): 113-125.
Ridwan, E. 2013. Etika Pemanfaatan Hewan Percobaan
dalam Penelitian Kesehatan. J Indon Med Assoc Vol. 63
(3).
Rosadi, B., Teguh, S., & Fachroerrozi, H. (2018).
Identifikasi Gangguan Reproduksi pada Ovarium Sapi
Potong yang Mengalami Anestrus Postpartum Panjang.
Jurnal Veteriner Vol.19 (3): 385-389.
Safitri, E., Tatik, H., Suzanita, U., Sri, M., Djoko, L. (2012).
Penurunan Estrus dan Gambaran Histopatologis
Ovarium Mencit (Mus musculus) Betina Kondisi
Malnutrisi. Veterinaria Medika Vol. 5(3): 207-214.
Siregar, T.N. (2010). Fisiologi Reproduksi Hewan Betina.
Syiah Kuala University Press, Banda Aceh. Halaman:
25-39.
Sutiyono, Daud S., Alam S. 2017. Identifikasi Gangguan
Reproduksi Sapi Betina di Peternakan Rakyat. Jurnal
Veteriner Vol.18 No.4: 580-588.
Wang, H., Hakan E., Lena S. 2000. Estrogen Receptors a
and b in the Female Reproductive Tract of the Rat
During the Estrous Cycle. Biology for Reproduction 63:
1331-1340.
Yahya, M. I. 2017. Tingkat Kejadian Gangguan Reproduksi
Ternak Sapi Perah di Kabupaten Enrekang [Skripsi].
Fakultas Peternakan. Universitas Hasanuddin.
Young, J. M., McNeilly, A. S. 2010. Theca : The Forgotten
Cell of the Ovarian Follicle. Society for Reproduction
and Fertility 140: 489-504.
ICAMBBE 2019 - 6th ICAMBBE (International Conference on Advance Molecular Bioscience Biomedical Engineering) 2019
48
