
Evaluation of Filling in the Hospital Laboratory Critical Value
Report:
The Collaborative Role of Laboratory Personnel and Nurses
Elsa Roselina and Intan Ananda Putri
Hospital Administration of Vocational Education Program, Universitas Indonesia,
Kampus Baru UI Depok 16424, Indonesia
Keywords: Laboratory Critical Value, Laboratory Personnel, Nurse, Collaboration, Hospital.
Abstract: This paper focused on the evaluation of filling in the hospital laboratory critical value report. This research
used mix methods, which is conducted in March and April 2019 at the "Z" hospital in the quality, laboratory
and nursing units. Quantitative data were taken from secondary data (laboratory critical value reports in
January and February 2019) and qualitative data sourced from in-depth interviews with 3 laboratory personnel
and 3 nurses. The research findings showed that reports on the results of laboratory critical values cannot be
completed, which is 29.5% (in January 2019) and 14.3% (in February 2019). The critical laboratory response
time was not up to standard, which is 71.0% (in January 2019) and 66.7% (in February 2019). The
achievement of the critical value reporting standard in January and February 2019 was 23.0%. The results of
in-depth interviews showed that obstacles in reporting were SOP, negligence in filling out, communication
with doctors and the role of nurse collaboration with laboratory staff. Collaboration between nurses and
laboratory staff and revision of the SOP for reporting laboratory critical values are needed in filling out
laboratory critical value reports.
1 INTRODUCTION
Laboratory services are part of hospital services. This
service is important to sustain the diagnosis,
treatment of diseases, and health recovery. Any errors
in the follow-up of laboratory results will cause
delayed treatment, medication errors, which have an
impact on patient safety.
George D. Lundberg introduced the term
"laboratory critical value" which was originally
called "the value of panic", more than 45 years ago,
defined as "pathophysiological conditions that can be
life-threatening unless something is done
immediately and corrective action is taken"
(Lundberg in Doering, Plapp and Crawford, 2014).
The critical value of the laboratory becomes very
important in protecting patient safety. Reporting
abnormal test results encourages early intervention in
the course of the disease with the intention of
stopping or hindering the process of the severity of a
disease.
Reporting critical values is a mandatory practice
in laboratory procedures, especially after the
inclusion of this activity in accreditation and clinical
laboratory certification programs (Priva, Sciacovelli,
Zaninotto, Laposata, Plebani, 2009).
The important role of laboratory critical values
makes it one of the requirements for hospital
accreditation both as an international and national
requirement. In the case of an international laboratory
accreditation program, timely reporting of critical
values from test results and diagnostic procedures is
the second target of accreditation undertaken to
improve the effectiveness of communication among
caregivers. As for some of the performance elements
measured, including by whom and to whom the
critical results are reported, the acceptable length of
time between the availability and reporting of critical
results and the timely evaluation of reporting critical
results (The Joint Commission, 2019).
In Indonesia, laboratory services are part of the
fifth standard of patient assessment, wherein standard
5.3.2 there are procedures for reporting critical
laboratory results. These procedures include the
determination of critical laboratory results and
threshold critical values for each type of test for each
Roselina, E. and Putri, I.
Evaluation of Filling in the Hospital Laboratory Critical Value Report: The Collaborative Role of Laboratory Personnel and Nurses.
DOI: 10.5220/0009571501590164
In Proceedings of the 1st International Conference on Health (ICOH 2019), pages 159-164
ISBN: 978-989-758-454-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
159
existing laboratory service, by whom and to whom
critical laboratory results must be reported, including
the time the results are delivered, recording and
establishing monitoring methods that meet the
provisions. The elements of the assessment are: there
are regulations regarding critical laboratory results,
reported by whom and to whom and the follow-up,
recording critical laboratory results recorded in the
patient's medical record, evidence of follow-up from
the reporting, and evidence of the implementation of
evaluation and follow-up to the entire process in order
to meet the provisions and modified as needed
(Komisi Akreditasi Rumah Sakit RI, 2017).
Observations at the "Z" hospital in February 2019
showed that there were several problems regarding
the reporting of laboratory critical values, including
the lack of confirmation of reporting critical values
from nurses to physicians responsible for service to
laboratory units, the incompleteness of filling
laboratory critical value reporting books and critical
value reporting responses. which is not in accordance
with the established standards. Based on this, then
this paper is focused on the evaluation of filling in the
hospital laboratory critical value reports including
regarding the collaborative role of laboratory
personnel and nurses.
2 METHOD
This research used a mixed-method, conducted in
March and April 2019 at the "Z" hospital, a type B
hospital in West Java. The quantitative data in this
study were secondary data sourced from the reporting
book and the critical value worksheet of the
laboratory in January and February 2019 in the
quality unit. This data is used to assess the
completeness of reporting. Quantitative data was also
sourced from medical record files, which are used to
view the response time of physicians in charge of
services. To obtain accurate qualitative data, the
triangulation of methods and sources is carried out.
Method triangulation carried out by combining the
method of in-depth interviews with document review.
Triangulation of sources is by using different
informants consisting of (1) head of laboratory
installation service, (2) head of laboratory
installation, (3) person in charge of laboratory, (4)
head nurse of inpatient care, (5) nurse in charge of
ICU and (6) nurse in charge of emergency
installation. The univariate analysis used in this study.
3 RESULTS AND DISCUSSION
The laboratory critical value report at the "Z" Hospital
in the first two months of 2019 totaled 65 reports: in
January 2019 there were 44 and in February 2019
there were 21.
3.1 Completion of Reporting the
Critical Value of "Z" Hospital
The completion of the critical value report can be seen
from three components: (1) the time received from
the results of the critical value, (2) the time of the
critical value report to the nurse, and (3) the time of
the critical value report from the nurse to the doctor
in charge of the service.
For the percentage of time received from the
results of critical values that were not filled either in
January 2019 or in February 2019, it was almost the
same: 4.5% and 5.0%. The percentage of time
reported critical value to nurses who did not do the
filling was higher in February 2019 (14.3%) than in
January (9.1%). The percentage of non-filling of the
critical time report from the nurse to the doctor in
charge of service in the two months was the same:
100.0%. More complete can be seen in table 1 and
table 2.
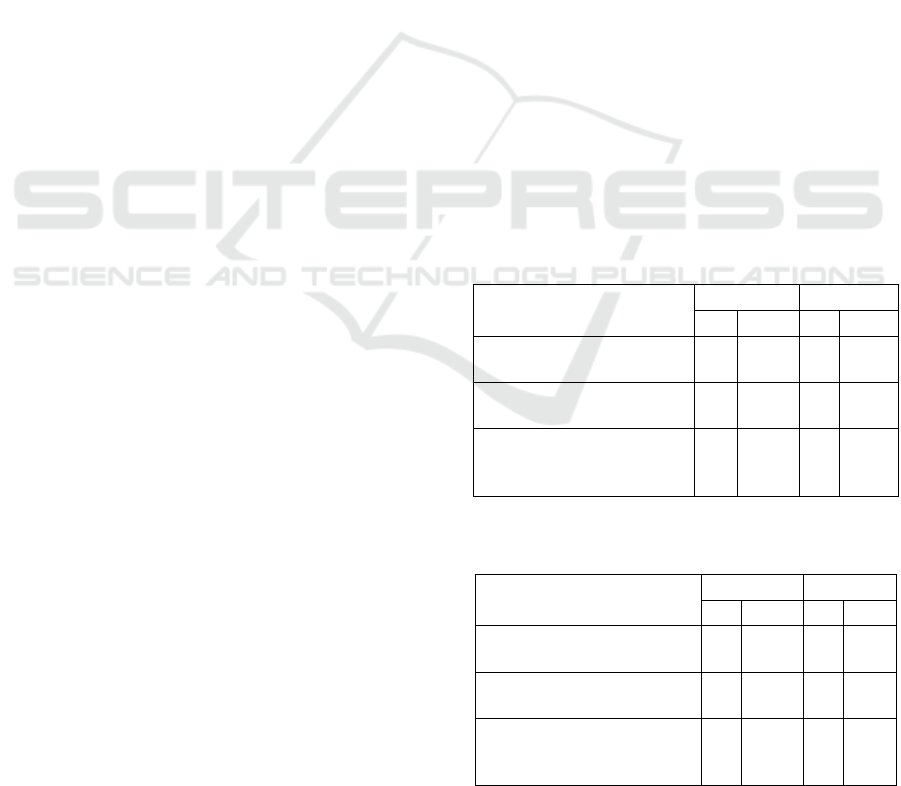
Table 1: Completion of reporting the critical value of "Z"
hospital in January 2019 (N = 44).
Filling Components
No Yes
n % n %
Time received from the
results of the critical value
2 4.5 42 95.5
Time report critical value
to the nurse
4 9.1 40 90.9
Time report critical value
from nurse to doctor in
charge of service
44 100,0 - -
Table 2: Completion of reporting the critical value of "Z"
hospital in February 2019 (N = 21).
Filling Components
No Yes
n % n %
Time received from the
results of the critical value
1 5.0 20 95.0
Time report critical value to
the nurse
3 14.3 18 85.7
Time report critical value
from nurse to doctor in
charge of service
21 100.0 - -
ICOH 2019 - 1st International Conference on Health
160
3.2 Report on the Critical Value of "Z"
Hospital That Can Be Analyzed
So that the critical value report can be analyzed, the
next step is to complete it through the medical record
file. This is done to fill the nurse's report hours to the
doctor in charge of the service.
The proportion of reports that ultimately could not
be analyzed because the hours reported to the doctor
could not be completed, more in January 2019 (as
much as 29.5%) than in February 2019 (as much as
14.3%). This can be seen in table 3.
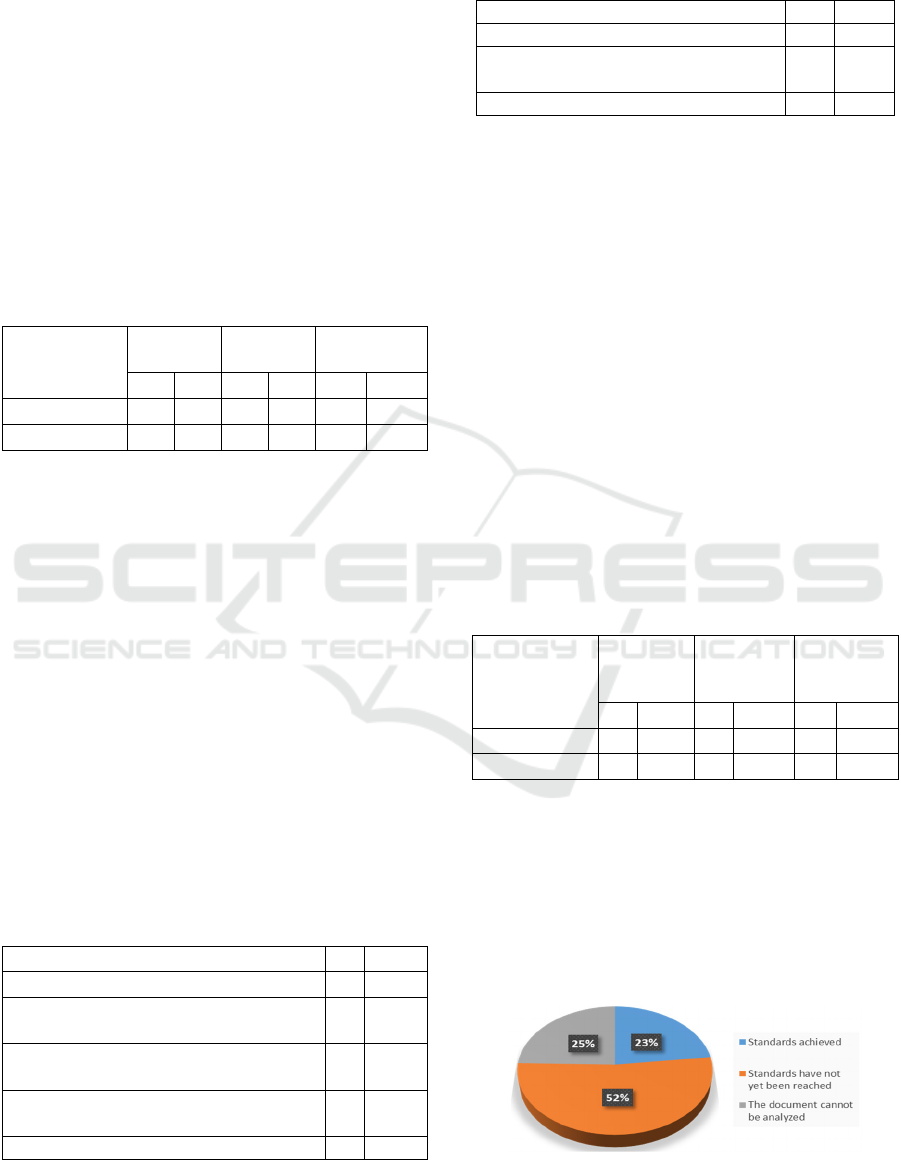
Table 3: Comparison of reports on the critical value of "Z"
Hospital which can be analyzed in January and February
2019.
Month
Can’t be
analyzed
Can be
analyzed
Total
n % n % N %
January 2019
13 29.5 31 70.5 44 100.0
February 2019
3 14.3 18 85.7 21 100.0
3.3 Incomplete Medical Record File at
"Z" Hospital
There were 4 reasons why the critical value reporting
book cannot be completed for the reporting hours of
the doctor in charge of the service: (1) the medical
record file was incomplete, (2) the incompatibility of
the medical record number in the reporting book, (3)
the medical record number in the book the report was
not complete, and (4) the medical record number was
not written in the reporting book.
The majority of causes cannot be completed in the
critical value reporting book for the report hours of
the physician in charge of services due to incomplete
medical record files, both in January 2019 (53.8%)
and February 2019 (66.7%). More complete can be
seen in table 4 and table 5.
Table 4: Incomplete medical record file at "Z" hospital in
January 2019 (N = 13).
Reasons
n
%
The medical record file was incomplete
7
53.8
The incompatibility of the medical record
number in the reporting book
2
15.4
The medical record number in the book
the report was not complete
1
7.7
The medical record number was not
written in the reporting book
3
23.1
Total 13 100.0
Table 5: Incomplete medical record file at "Z" hospital in
February 2019 (N = 3).
Reasons
n
%
The medical record file was incomplete
2
66.7
The medical record number was not
written in the reporting book
1
33.3
Total
3
100.0
3.4 Response Time Reports on
Laboratory Critical Values at "Z"
Hospital
After the report can be analyzed through the medical
record file, an evaluation of the response time of the
laboratory's critical values will be assessed. The "Z"
hospital sets the standard that a critical value must be
reported to the person in charge of the service in order
to receive further treatment instructions in less
than/equal to 30 minutes. "Z" hospital has set a target
of achieving 100% of the response time reporting
critical values.
In general, the response time from the laboratory
critical value report did not reach the standard (more
than 30 minutes), both in January 2019 (71.0%) and in
February 2019 (66.7%). This can be seen in table 6.
Table 6: Comparison of achievement of targets in the
response time of reporting laboratory critical values at "Z"
hospital in January and February 2019.
Month
Not
achieved
(> 30 mnt)
Achieved
(≤ 30 mnt)
Total
n % n % N %
January 2019
22 71.0 9 29.0 31 100.0
February 2019
12 66.7 6 33.3 18 100.0
3.5 Achievement of Standards in
Reporting Laboratory Critical
Values in "Z" Hospital
Based on the exposure of previous quantitative data,
it can be stated that the achievement of the critical
value reporting standard in January and February
2019 was 23.0%. This can be seen in Figure 1.
Figure 1: The achievement of the critical value reporting
standard in January and February 2019.
Evaluation of Filling in the Hospital Laboratory Critical Value Report: The Collaborative Role of Laboratory Personnel and Nurses
161

The results of this study are similar to the research
conducted by Adiputra (2014) in Bali, where the
critical value reporting rate in Sanglah Hospital
Denpasar was 30.01%.
3.6 Reporting Process
3.6.1 Reporting Steps
The person in charge of the laboratory and the head
of the laboratory service explained about the critical
value report steps: the results come out, several stages
of analysis are carried out, the laboratory officer
forwards the report to the nurse, then the nurse reports
to the doctor in charge of the service.
"...first seen pre-analytic, analytic and post-
analytic, it means whether we have correct sampling,
if it is correct we report it to the person in charge of
our clinical laboratory or pathology doctor, for
example, the 3rd or 4th floor of a child, we report to
the nurse, later it will be reported, confirmed
continue to report back hours recorded in the
reporting book, after 15 minutes we followed up what
patients were taken or what drugs were given, if the
outpatients he enrolled to our doctor, we immediately
inform the nurse or doctor if the outside patient we
report later to the emergency room doctor later the
emergency room doctor will take action..." (person in
charge of the laboratory)
"...critical values are immediately reported to me,
then I agree, then the laboratory staff will report to
the nurse, and the nurse must report to the
responsible doctor..." (head of laboratory services)
The results of this interview are in accordance
with hospital accreditation rules set by the Indonesian
Hospital Accreditation Commission (Komisi
Akreditasi Rumah Sakit RI, 2017) which is the
hospital sets regulations to carry out laboratory
quality control procedures, are evaluated and
recorded as documents. The quality control program
includes the Pre-analytic, Analytic and Post-analytic
stages which include validation of tests used for tests
of accuracy, precision, results of range of values.
3.6.2 Reporting Time
The person in charge of the laboratory and the head
of the laboratory installation stated that the results of
the critical values are consulted to the clinical
pathologist and are reported immediately.
"...It must be reported immediately, yes it must be
checked by PJ, the pre-analytical sampling is correct
or not, if it is correct, we report immediately but must
first report to the clinical pathologist, the principal
should be reported immediately…" (laboratory
person in charge)
"... After knowing that there is a critical value, we
have to see what the pre-analytic looks like, we will
find out first how it's taken. If everything is correct
and there is no doubt, we consult with the clinical
pathologist and report it directly to the relevant
unit..." (head of the laboratory installation).
This is consistent with the definition of the critical
test stated by Campbell et al. (2015) as "tests that
require direct communication regardless of whether
the results are normal, significantly abnormal or
critical".
3.6.3 Initial Report from the Laboratory
The person in charge of the laboratory stated that in
the Standard Operating Procedures (SOP) the
reporting is done by the person in charge, but if the
person in charge is busy can be reported by the
laboratory analyst after reporting to the person in
charge first.
"...Reporting is done by all laboratory analysts if
the person in charge is busy but still reports to the
person in charge first. So, the reporter does not have
to be in charge of the laboratory. In the SOP it should
be noted that the person in charge should be, but in
real conditions, it cannot be done because the person
in charge is busy..." (Laboratory person in charge).
3.6.4 Human Resources in the Laboratory
The person in charge of the laboratory stated that
human resources in carrying out work in the
laboratory coupled with critical monitoring and
reporting of critical value were still lacking.
"...Human resources are lacking, for example,
inpatients we do sampling only 1 person for 3 floors
of inpatients. This is lacking. Even though we are
required to process this critical value quickly. If the
service is to be good, there must be a lot of human
resources...” (laboratory person in charge).
3.7 Reporting Constraints
3.7.1 SOP of Laboratory Critical Value
According to the laboratory personnel, the SOP for
reporting critical values has been socialized.
"...Already, through hand-over shift..." (the
person in charge of the laboratory).
"...Already for us, for example, there is a critical
value in hematology he must immediately
understand..." (head of laboratory installation
services).
ICOH 2019 - 1st International Conference on Health
162

"...SPO for all nursing must know and for the
laboratory, every new employee will definitely be
socialized..." (head of the laboratory installation).
Otherwise, according to nurses, in general, they
have never read a critical value SOP, those who have
read only once. There were also those who say the
SOP socialization is lacking.
"...Never read, SOP has never been in an
emergency department..." (nurse in charge of
emergency installation)
"...I knew I've read it once, it's just lacking in
socialization..." (the head nurse of inpatient care).
"...I have never read SOP. The SOP has been
socialized and I have ever heard..." (nurse in charge
of ICU).
3.7.2 Negligence
According to the laboratory personnel, negligence is
one of the reasons for no report hours in the laboratory
critical value reporting book.
"...Forgetting, in a hurry or negligent because of
doing other things..." (the person in charge of the
laboratory).
"...That's because the officers who might be
negligent..." (head of the laboratory installation).
3.7.3 Communication with Doctor in Charge
of Service
According to laboratory personnel, monitoring of the
hours reported to the doctor in charge of the service
is carried out by nurses. They cannot monitor and
carry out a follow-up.
"...We cannot monitor the communication from
the nurse to the doctor in charge. Whether the doctor
receives the results immediately or the next day, we
don't know. So the rules regarding critical values can
only be monitored and applied in laboratory units..."
(head of laboratory installation services).
"... For the time reported to the doctor the nurse
should have informed us because we could not afford
to have to follow up there..." (head of the laboratory
installation).
The nurses stated that the obstacle in
communicating with the doctor in charge as they were
difficult to contact.
"...The doctor can't be contacted..." (nurse in
charge of emergency installation).
"... Sometimes the doctor is hard to contact,
sometimes the doctor is in a meeting, many of the
doctors are from other hospitals as well, so we report
to the doctor's office first so that we can respond
immediately ..." (the head nurse of inpatient care).
"... Usually, the doctor can be contacted except at
night, but we will still contact if we cannot the next
morning, but still report the doctor on duty to get
instructions..." (nurse in charge of ICU).
The communication problems experienced by the
"Z" hospital are similar to those experienced at
Sanglah hospital in Bali, where many doctors in
charge of services cannot be contacted (Adiputra,
2014).
3.7.4 Nurse Communication with
Laboratory Personnel
The nurses stated that the confirmation to the
laboratory staff was only done in reading the results
of the laboratory. They did not confirm the results of
the reporting to the doctor in charge of the service.
There was also a statement that implies that the
laboratory should follow up with the nurses
indirectly. In addition, it may be because nurses
forgot to report to the laboratory staff.
"...No, we won't tell the lab anymore, at most we
just confirm it, is true about the results of laboratory,
after that we reported the doctor or the emergency
room doctor, that's it..." (nurse in charge of
emergency installation).
"...Maybe if the data is indeed needed by the lab,
they have never followed up to us about what we get
from the doctor..." (the head nurse of inpatient care).
"...I've heard, but nurses often forget to report
back to the laboratory staff..." (nurse in charge of
ICU).
4 CONCLUSIONS
The completion of laboratory critical value reports
has not optimal yet. These are due to the
incompleteness of filling out the report book, the
results of laboratory critical values that cannot be
completed, the response time of critical values that do
not meet standards, laboratory staff are negligent in
filling out report hours, nurses who do not know or
who have never read the SOP, nurses who do not
know not knowing that the results of instructions
from the doctor in charge must be reported back to the
laboratory unit, and the doctor in charge who is
difficult or cannot be contacted.
SOP for reporting critical laboratory values need
to be socialized for nurses. A revision of the SOP for
reporting laboratory critical values is needed: details
of who should be followed up to get instructions from
the physician in charge of service and adding a flow
Evaluation of Filling in the Hospital Laboratory Critical Value Report: The Collaborative Role of Laboratory Personnel and Nurses
163

of critical value reporting. It also requires the addition
of a critical value validation time column. Therefore,
the collaboration between nurses and laboratory staff
is needed in filling out laboratory critical value
reports.
REFERENCES
Adiputra, N.S., (2014). Analisis manajemen pelaporan
nilai kritis di laboratorium patologi klinik RSUP
Sanglah Denpasar. Thesis Public Health Faculty
Universitas Indonesia. [unpublished]
Campbell C, Caldwell G, Coates P, et al. (2015). Consensus
Statement for the Management and Communication of
High Risk Laboratory Results. Clin Biochem Rev, Vol.
36, p.97-105. Available at: https://www.ncbi.nlm.nih.
gov/pmc/articles/PMC4745612/pdf/cbr-36-97.pdf.
[Accessed 2 September 2019].
Doering, T.A., Plapp, F. and Crawford, J.M, (2014).
Establishing an Evidence Base for Critical Laboratory
Value Thresholds. American journal of clinical
pathology, [online] Vol. 142, p.617. Downloaded from
https://academic.oup.com/ajcp/article-abstract/142/5/
617/1760784 by Universitas Indonesia user [Accessed
1 September 2019].
Komisi Akreditasi Rumah Sakit RI, (2017). Standar
nasional akreditasi rumah sakit, edisi 1. Available at:
http://www.pdpersi.co.id/kanalpersi/manajemen_mutu
/data/snars_edisi1.pdf. [Accessed 1 September 2019].
Priva E, Sciacovelli L, Zaninotto M, Laposata M, Plebani
M, (2009). Evaluation of effectiveness of a compute-
rized notification system for reporting clinical values.
Am J Clin Pathol, Vol. 131, p. 432-441. Available at:
https://www.researchgate.net/publication/
24026541_Evaluation_of_Effectiveness_of_a_Compu
terized_Notification_System_for_Reporting_Critical_
Values. [Accessed 2 September 2019].
The Joint Commission, (2019). National patient safety
goals effective January 2019: laboratory accreditation
program. Available at: https://www.jointcommission.
org/ASSETS/1/6/NPSG_CHAPTER_LAB_JAN2019.
PDF. [Accessed 1 September 2019].
ICOH 2019 - 1st International Conference on Health
164
