
The Peak of Cytochrome-c (Cyt-c) Gene Expression in Inflammatory
Stage after Amputation of Digit Tip Mice (Mus musculus)
Titta Novianti
1
, Febriana Dwi Wahyuni
1
, It Jamilah
2
and Syafruddin Ilyas
2
1
Department of Biotechnology, Universitas Esa Unggul, Jl. Raya Arjuna Utara No. 9, Jakarta Barat, Indonesia
2
Department of Biology, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Cytochrome-c (Cyt-c), Apoptosis, Mitochondria, Tissue Regeneration.
Abstract: The complexity of the tissue regeneration process requires the activity of cells and energy. The energy
obtained from cellular respiration processes. The cellular respiration involves the enzyme has a role transfer
an electron. Cytochrome-c (Cyt-c) is an enzyme in the mitochondria inner membrane that has a role in cell
respiration and stimulates apoptosis cells. We studied the Cyt-c gene expression in inflammatory phase of
tissue regeneration of digit tip mice (Mus musculus) after amputation to analyze a role in tissue regeneration.
The result of the Cyt-c gene expression reached a peak on day 3. It showed that the tissue regeneration process
needs high energy and involves cell apoptosis to stimulate regeneration. The result of ANOVA homogeneity
test of Cyt-c gene expression differentt significantly for each growth day (p <0.05).
1 INTRODUCTION
The tissue regeneration process involves the forming
of new cells and tissue. The damaged cells will
undergo a process of apoptosis that the programmed
of cell death (Gauron et al., 2013). Apoptosis cell is
interested in the tissue regeneration process because
it is related to the formation of new cells. The
macrophages will be phagocytosed into the dead cells
and stimulate extracellular matrix formation that
plays a role in tissue regeneration (Reinke & Sorg,
2012).
The higher the apoptotic cell process occurs, the
higher the extracellular matrix produced (Calve &
Simon, n.d.). An apoptosis, cell process involving the
enzymes and proteins, one of which is cytochrome c
(Allen, 2011). This enzyme has a role in electron
transfer from system III to system IV in mitochondria
inner membrane and a role in cell apoptosis. The Cyt-
c biogenesis process will increase the Cyt-c enzyme
in cell apoptosis. A Cyt-c biogenesis process is a
multiplication of the Cyt-c enzyme (Panigrahy et al.,
2013).
The process of tissue regeneration occurs in four
phases, the wound-healing, the blastema, the
regeneration phase, and the materials phase (Krafts,
2010). The tissue was dominated by the white blood
cells in the wound-healing phase. The macrophage
phagocytes the antigen and phagocytes the apoptotic
cells in the wound-healing phase. The wound-healing
phase occurs in the first days after injury (Mescher,
2017).
In this study, we will analyze the Cyt-c gene
expression in tissue regeneration of digit tip mice
post-amputation. The study result to be the initial
research for further analysis stimulates the
regeneration process in organisms that have limited
ability in tissue regeneration.
2 MATERIAL AND METHOD
2.1 Sample
The research ethics code for this study proposed by
the Research Ethics Commission Esa Unggul
University. The research sample was thirty male mice
(Mus musculus) for Swiss Webster that eight weeks
old and weighing twenty grams. The number of
samples is adopted by the Federer formula. We got
the mice from the research and development
laboratory, the Ministry of Health of the Republic of
Indonesia. The sample was maintained and treated by
the laboratory assistant from the Health Ministry of
the Republic of Indonesia. Mice were anesthetized by
ketamine/xylazine at a dose of 0.5 gr/kg BW and
78
Novianti, T., Wahyuni, F., Jamilah, I. and Ilyas, S.
The Peak of Cytochrome-c (Cyt-c) Gene Expression in Inflammatory Stage after Amputation of Digit Tip Mice (Mus musculus).
DOI: 10.5220/0009566300780082
In Proceedings of the 1st International Conference on Health (ICOH 2019), pages 78-82
ISBN: 978-989-758-454-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
amputated on the 3rd phalanges of digit tip mice. The
tissue growth on day 0 (4 hours after amputation), day
1, day 3, and day 5 after the amputation was collected
to analyze the sample for gene expression and
histological analysis.
2.2 Histology Preparation
Histology preparations stained with hematoxylin-
eosin (HE) strokes and histochemical: 10% formalin;
70% alcohol; 80% alcohol; 95 % alcohol; and 100%
alcohol; xylol; paraffin block; hematoxylin-eosin;
equates; the outward appearance of Van Gieson.
Software ImageJ I-46 has various features to
analysis the semi-quantitative of the histological
sample of HE staining. Image J software can use to
calculate the number of cells and measure the length
or area of cells and tissues. Image J software can be
downloaded for free and used offline.
The length calculation is done by opening the
histology image file first and setting the image scale
using the scale set feature. The length calculation
used in the line drawing feature is got automatically
by using the measure feature.
2.3 Analysis of Gene Expression
The primary DNA of the Cyt gene and the 18S gene,
as a reference gene, were amplified by qPCR
procedure. Desain primer Cyt-c gene Forward
ATTCCTTCATGTCGGACGAG and Reverse
ACTGAGAAGCCCCCTCAAAT.
The qPCR method amplification DNA through
the stages is DNA synthesis, reverse transcriptase,
amplification with 40 cycles at an annealing
temperature of 55
0
C. Finally, the stage is the melting
curve stage. Negative controls operated by free water
as a substitute for RNA to get the incorrect positive
results. The results of qRT-PCR obtained the value of
efficiency and Cycle Threshold (CT) of DNA
amplification. Analysis of gene expression assessed
by relative qualification by the value of mRNA
expression quantification relatively by the Livak
method.
2.4 Statistical Analysis
Statistical analysis of the normality test used the
Kolmogorov Smirnov test. The data distributed not
normally, so analytical analysis using a non-
parametric test. The homogeneity test performed
using the ANOVA test and the correlation test by the
Spearman test.
3 RESULTS
3.1 The Growth of Digit Tip Mice
(Mus musculus)
After amputation, the digit tip mice grow to replace
the lost tissue (figure 1). On day 0 and day 1, therein
common is no visible growth of tissue. We suspected
that it is the inflammatory tissue. From day 3 to day
5, the tissue appeared to grow faster in the wound
area. We suspect that the cell was more actively
dividing. Growing tissue remains the collecting
dividing cells that form the new tissue.
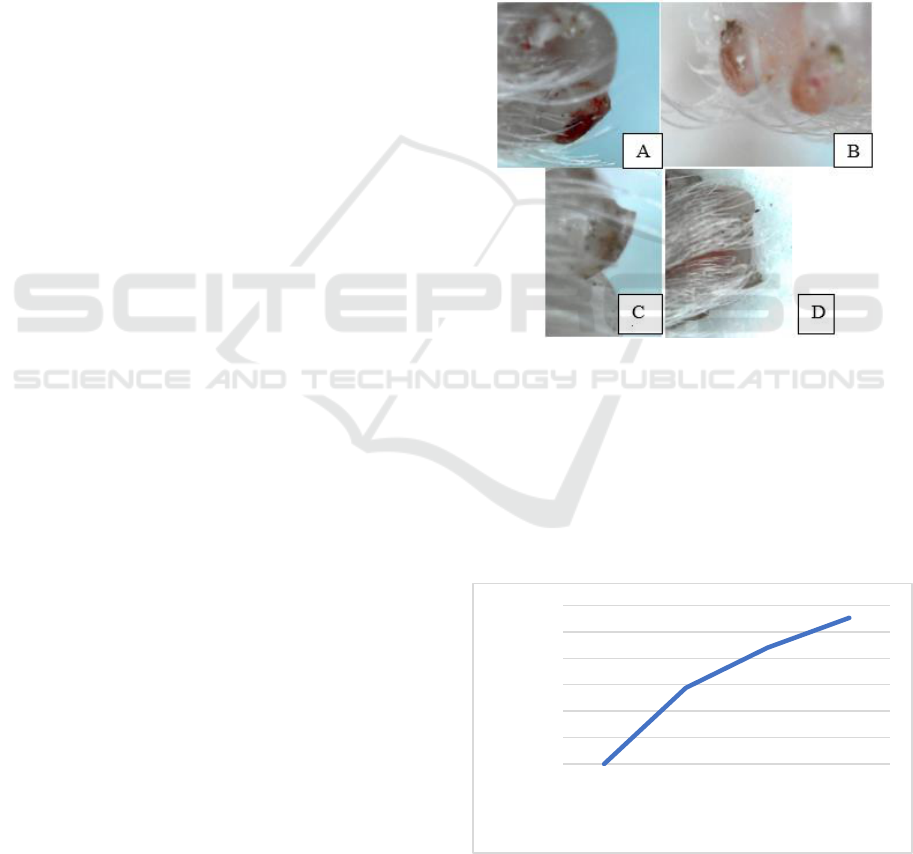
Figure 1: Growth of digit tip mice (Mus musculus) from day
0 to day 5 after amputation (A) Digit tip on day 0 (4 hours
after amputation) (B) 1 day after amputation (C) 3 day after
amputation (D) 5 day after amputation.
The growth curve of the digit tip mice after
amputation shown in fig 2. The growth curve grows
faster, significantly from day 0 (4 hours after
amputation) until day 5 after amputation. The growth
indicates that some cells divided actively.
Figure 2: Growth curve of a digit tip mice (Mus musculus)
from day 0 (4 hours after amputation) until day 5. Curve
lines increase rapidly from day 0 to day 5.
0
5
10
15
20
25
30
0 1 3 5
lengt of digit tip (mm)
growth day
The Peak of Cytochrome-c (Cyt-c) Gene Expression in Inflammatory Stage after Amputation of Digit Tip Mice (Mus musculus)
79
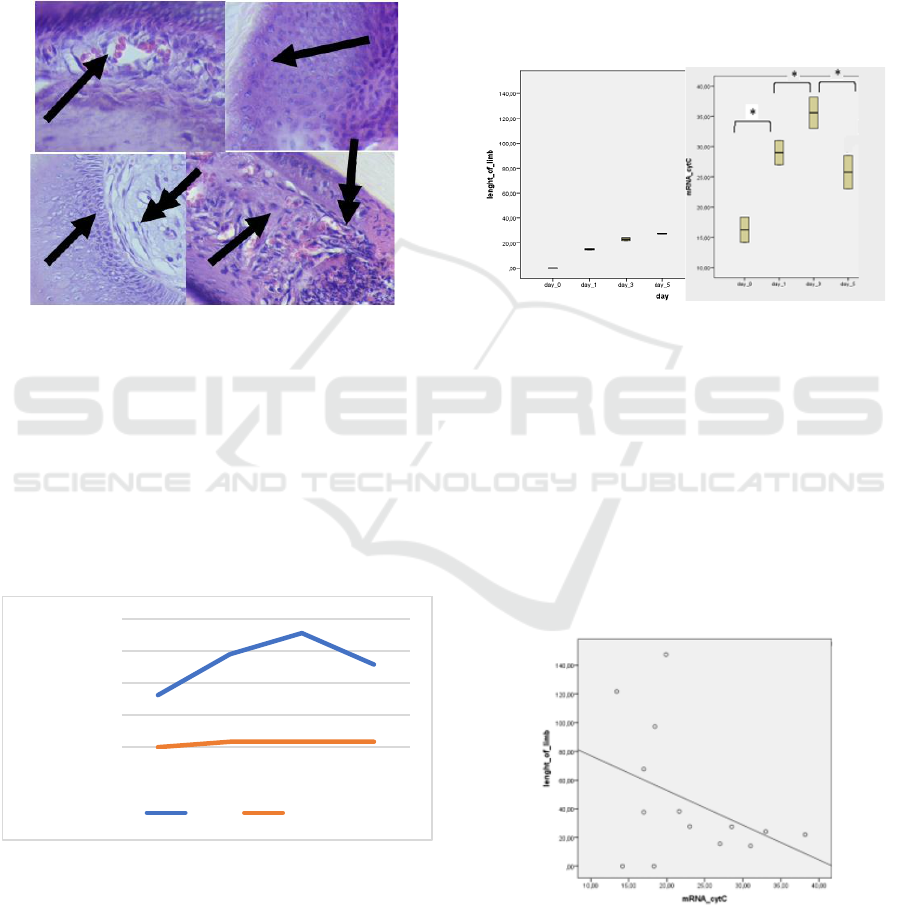
3.2 The Result of Histology Staining
The histological analysis of digit tip mice (Mus
musculus) shows the intense activity of cells in Figure
3. On day 0 (4 hours after amputation) and day 1, the
wound area tissue dominated by white blood cells. In
some of the wound area, there are red blood cells. On
day 5, stem cells divided and differentiated to form
the new tissue.
Figure 3: The Tissue histology of digit tip mice (Mus
musculus) (A) Tissue digit tip mice on day 0 shows the
presence of red blood cells (single arrow) that spread in the
tissue due to injury when amputation (B) day 1, white blood
cells that have many cell nuclei (single arrow) spread in the
area wound (C) osteoblast cells (single arrow) that are
actively dividing, fibroblasts like cells appear to start
spreading in fat tissue (D) cells like fibroblasts (single
arrows) division results begin to increase, the appearance of
new blood vessels (double arrows) (enlargement 400 x).
3.3 mRNA Gene Expression
Figure 4: Cyt gene mRNA expression relative to control.
The results of gene expression quantitative relatively
than controls produce diverse gene expression at each
stage of tissue regeneration (Figure 3). In the
inflammatory phase, the Cyt gene expression relatively
higher than controls. Cyt gene expression increased
and reached a peak on day 3 after amputation.
3.4 Statistical Analysis
3.4.1 Homogeneity Test
Homogeneity test results on the length of the digit tip
of mice on each growth day showed a significant
difference in tissue growth (p < 0.05) using the
ANOVA test. There was no difference in the growth
of digit tip tissue growth between day 0 to day 5. The
difference in the expression of the Cyt gene mRNA
was significantly different (p < 0.05) with the
ANOVA test.
A
B
Figure 5: Significantly different ANOVA homogeneity test
(p < 0.05) (A) there is no different growth of digit tip mice
(Mus musculus) from day 0 until day 5 (B) Cyt gene mRNA
expression that is different between day 0 and day 1,
between day 1 and day 3, between day 3 and day 5.
3.4.2 Correlation Test
Spearman correlation test results indicated there is no
correlation between the expression of Cyt gene
mRNA and the length of tip mice digit growth.
Spearman correlation test results showed p > 0.05.
Figure 6: Spearman's correlation test between the growth
length of digit tip mice with the mRNA expression of the
Cyt gene (p > 0.05).
0,00
10,00
20,00
30,00
40,00
0 1 3 5
mRNA expression relatively
than control
growth day
Cyt-c Control
ICOH 2019 - 1st International Conference on Health
80

4 DISCUSSION
The growth of the digit tip mice (Mus musculus)
tissue indicates a relatively rapid growth in the
inflammatory phase. The results of the histological
analysis showed the proliferation and differentiation
of cell activity. White blood and fibroblast-like cells
appear on day 5 Increaser than day 1. According to
Mechner, in inflammation phase occurs the process
of cleansing the tissue in the wound area from
bacterial infection and cells undergoing apoptosis by
white blood cells (Mescher, 2017).
This activity of cells requires high energy that it
will trigger cellular respiration in mitochondria to
produce energy (ATP) (Duguez, Féasson, Denis, &
Freyssenet, 2002). Cytochrome-c (Cyt-c) is a protein
in the inner membrane of mitochondria that play a
role in capturing electrons in the respiration chain and
acts as a deterrent and inhibits oxidative stress (Allen,
2011). The activity of cells requires high energy that
will trigger cellular respiration in mitochondria to
produce energy like ATP (Pelicano et al., 2003).
Cytochrome-c (Cyt-c) protein is in the inner
membrane mitochondria has a role in capturing of
electrons, acting as a deterrent, and inhibiting
oxidative stress. The other role of Cyt-c enzyme, it
acts as an agent in the process of cell apoptosis (Allen,
2011); (Wright et al., 2007). The BCL-2 protein gives
the signal to Cyt-c and stimulates the caspase enzyme
in the process of apoptosis occurs (Allen, 2011).
In tissue regeneration of digit tip mice, the
expression of Cyt-c gene is relatively high and
reaches its peak in the inflammatory phase. We
suspect that this expression is thought to be due to the
role of Cyt-c in cellular respiration and the process of
cell apoptosis.
Awarding to Osuma, there was an increase in
energy demand that increased during the tissue
regeneration process (Osuma, Riggs, Gibb, & Hill,
2018). The analysis of Cyt-c gene expression
demonstrated that an increase in gene expression in
the inflammatory phase accompanied by an increase
in cell differentiation and proliferation. It's suspected
that in this phase there was an increase in energy
demands and increasing cell apoptosis process.
Awarding to Bergmann and Steller, the process of cell
apoptosis will stimulate the proliferation and
differentiation of stem cells and progenitor cells in
tissues (Akhmetshina et al., 2012). The histological
analysis of digit tip mice tissue regeneration showed
an increase in cell proliferation and differentiation
after the severe of the Cyt-c gene expression. In the
tissue, there is a proliferation of basal lamina cells,
white blood cells, fibroblast-like cell cells, and the
cells of dermis tissue. Activation of proliferation and
differentiation of progenitor cells in the inflammatory
phase produces the new blood cells and the layer of
epidermis and dermis so that the wound area begins
to cover.
The study results can be used as a reference for
the next study about the stimulation of adult tissue
regeneration that has limited ability in tissue
regeneration. To stimulate adult tissue regeneration,
we must try stimulating the expression of genes that
play a role in overcoming inflammation, genes that
play a role in providing energy, and genes that play a
role in the process of proliferation, differentiation,
cell migration, and tissue morphogenesis.
5 CONCLUSIONS
Cyt-c gene expression occurs in the inflammatory
phase that stimulates the activity of cell proliferation
and differentiation.
ACKNOWLEDGMENT
Thank you to the Ministry of Higher Education and
Higher Education for the PKPT Scholarship Research
Grant for the next two years (2019-2020). Thank you
to Esa Unggul University for supporting both morally
and materially for doing this research as well.
REFERENCES
Akhmetshina, A., Palumbo, K., Dees, C., Bergmann, C.,
Venalis, P., Zerr, P., … Distler, J. H. W. (2012).
Activation of canonical Wnt signalling is required for
TGF-β-mediated fibrosis. Nature Communications, 3.
https://doi.org/10.1038/ncomms1734
Allen, J. W. A. (2011). Cytochrome c biogenesis in
mitochondria - Systems III and v. FEBS Journal,
278(22), 4198–4216. https://doi.org/10.1111/j.1742-
4658.2011.08231.x
Calve, S., & Simon, H. (n.d.). Biochemical and mechanical
environment cooperatively regulate skeletal muscle
regeneration. https://doi.org/10.1096/fj.11-200162
Duguez, S., Féasson, L., Denis, C., & Freyssenet, D. (20
02). Mitochondrial biogenesis during skeletal muscle
regeneration. American Journal of Physiology.
Endocrinology and Metabolism, 282(4), E802–E809.
https://doi.org/10.1152/ajpendo.00343.2001
Gauron, C., Rampon, C., Bouzaffour, M., Ipendey, E.,
Teillon, J., Volovitch, M., & Vriz, S. (2013). Sustained
production of ROS triggers compensatory proliferation
The Peak of Cytochrome-c (Cyt-c) Gene Expression in Inflammatory Stage after Amputation of Digit Tip Mice (Mus musculus)
81

and is required for regeneration to proceed. Scientific
Reports, 3, 1–9. https://doi.org/10.1038/srep02084
Krafts, K. P. (2010). The hidden drama Tissue repair.
Organogenesis, 6(4), 225–233. https://doi.org/10.4161/
org6.4.12555
Mescher, A. L. (2017). Macrophages and fibroblasts during
inflammation and tissue repair in models of organ
regeneration, (March), 39–53. https://doi.org/10.1002/
reg2.77
Osuma, E. A., Riggs, D. W., Gibb, A. A., & Hill, B. G.
(2018). High throughput measurement of metabolism in
planarians reveals activation of glycolysis during
regeneration. Regeneration, (August), 1–9.
https://doi.org/10.1002/reg2.95
Panigrahy, D., Kalish, B. T., Huang, S., Bielenberg, D. R.,
Le, H. D., Yang, J., … Kieran, M. W. (2013).
Epoxyeicosanoids promote organ and tissue
regeneration. Proceedings of the National Academy of
Sciences, 110(33), 13528–13533. https://doi.org/10.
1073/pnas.1311565110
Pelicano, H., Feng, L., Zhou, Y., Carew, J. S., Hileman, E.
O., Plunkett, W., … Huang, P. (2003). Inhibition of
Mitochondrial Respiration. Journal of Biological
Chemistry, 278(39), 37832–37839. https://doi.org/10.
1074/jbc.M301546200
Reinke, J. M., & Sorg, H. (2012). Wound repair and
regeneration. European Surgical Research, 49(1), 35–
43. https://doi.org/10.1159/000339613
Wright, D. C., Han, D. H., Garcia-Roves, P. M., Geiger, P.
C., Jones, T. E., & Holloszy, J. O. (2007). Exercise-
induced mitochondrial biogenesis begins before the
increase in muscle PGC-1?? expression. Journal of
Biological Chemistry, 282(1), 194–199.
https://doi.org/10.1074/jbc.M606116200
ICOH 2019 - 1st International Conference on Health
82
