
Enhanced Extraction of Total Polyphenols Content from Mitragyna
Speciosa (Korth.) Havil Leaves using the Natural Deep Eutectic
Solvent-based Microwave-assisted Extraction Method
Islamudin Ahmad
1
, Wisnu Cahyo Prabowo
2
, Yuspian Nur
3
, Lulu Irawan
1
, Andi Yusniah
1
,
Bakti Puji Rahayu
1
, Ramila Hidayati
1
, Hesti Nurlinda
1
and Herman
3
1
Department of Pharmaceutical Sciences, Mulawarman University, Samarinda, Indonesia
2
Department of Vocational Pharmacy, Mulawarman University, Samarinda, Indonesia
3
Laboratory of Research and Development of FARMAKA TROPIS, Mulawarman University, Samarinda, Indonesia
andiyusniah80@yahoo.com, {luluirawan, bakti.rahayu31, ramilahidayati25, herman.mulawarman}@gmail.com,
hesti_nurlinda20@yahoo.co.id
Keywords: Microwave-assisted Extraction, Mitragyna Speciosa (Korth.) Havil, Natural Deep Eutectic Solvent,
Total Polyphenolic Content.
Abstract: Exploration of natural products is highly dependent on separation techniques, mainly solvent selection, one
of which is using the green chemistry principle approach. Mitragyna speciosa (Koth.) Havil is an endemic
plant of East Kalimantan which traditionally used for the treatment of various diseases. On the other hand,
this plant has an addictive effect. The study aims to determine the impact of using natural deep eutectic
solvent-based microwave-assisted extraction (NADES-MAE) on total polyphenol content (TPC) extraction
from M. speciosa. Natural deep eutectic solvent (NADES) made by malting two combination types include
citric acid-glucose; choline chloride-sorbitol; malic acid-glucose; and lactic acid-sucrose. The extraction
process was carried out using microwave-assisted extraction (MAE), and the determination of TPC was
analyzed using Folin-Ciocalteau’s reagent and measured with a spectrophotometer type including 246.70 mg
GAE/g sample (citric acid-glucose), 227.33 mg GAE/g sample (malic acid-glucose), 222.26 mg GAE/g
sample (lactic acid-sucrose), and 219.02 mg GAE/g sample (choline chloride-sorbitol). According to the
results, the NADES-MAE method shows differences in TPC based on the NADES types.
1 INTRODUCTION
Mitragyna speciosa [Korth.] Havil belongs to the
family Rubiaceae, which is an endemic plant of
Southeast Asia. It is spread in several countries such
as Thailand, Vietnam, Malaysia, and Indonesia
(Hassan et al., 2013). In Indonesia, this plant
commonly found on Kalimantan island, mainly in
East Kalimantan. Local community uses the leaves of
M. speciosa for traditional medicine either with
chewed up, smoked like cigarettes, and brewed like
tea.
M. speciosa leaves are traditionally believed to
have several medicinal properties such as a wound,
fever, muscle aches, reduce appetite and diarrhea
(Hassan et al., 2013; Raini, 2017). It has been
scientifically proven to have pharmacological effects
such as analgesic, stimulant, antidepressant, anti-
inflammatory, antinociceptive, antioxidant, and
antibacterial activities. (Mossadeq et al., 2009;
Parthasarathy et al., 2009; Luliana et al., 2018).
Besides, this plant is an export commodity for
farmers in East Kalimantan. Although most European
countries have banned their use because of the
addictive effects of the compounds they have, such as
mitragynine, 7-hydroxy-mitragynine, painantein,
speciesiin, and speciosiliatin (Horie et al., 2005;
Chittrakarn, Penjamras and Keawpradub, 2012;
Henningfield et al., 2018), this plant is also rich in
polyphenols, terpenoids, and several types of
glycosides (Takayama, 2004; Tohar et al., 2016;
Chittrakarn, Penjamras, and Keawpradub, 2012;
Brown, Lund, and Murch, 2017).
In some countries such as Malaysia, Thailand,
Myanmar, and Australia, this plant is illegal.
Meanwhile, New Zealand, Romania, Finland,
72
Ahmad, I., Prabowo, W., Nur, Y., Irawan, L., Yusniah, A., Rahayu, B., Hidayati, R., Nurlinda, H. and Herman, .
Enhanced Extraction of Total Polyphenols Content from Mitragyna Speciosa (Korth.) Havil Leaves using the Natural Deep Eutectic Solvent-based Microwave-assisted Extraction Method.
DOI: 10.5220/0009564500720077
In Proceedings of the 1st International Conference on Health (ICOH 2019), pages 72-77
ISBN: 978-989-758-454-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

Germany, and Denmark are controlled and included
in the Schedule 1 drug category (Saingam et al., 2013;
Henningfield et al., 2018).
The exploration of active compounds from natural
products is very dependent on the separation
technique. One way is the approach of green
chemistry principles. Natural deep eutectic solvent
(NADES) is a green solvent that can be an alternative
solvent to replace conventional organic solvents.
NADES has an advantage compared to conventional
solvents because it has low toxicity, biodegradability,
biocompatible with many media, and edible (Savi et
al., 2018; Gomez and Espino, 2018).
Some study have reported on the use of NADES
as an alternative solvent and combined with non-
conventional extraction methods (such as microwave,
supercritical, and ultrasonic) namely extraction of
caffeine and chlorogenic acid from coffee beans
(Ahmad et al., 2018), baicalin extraction from
Sturellaria baicalencis Gergi (Wang et al., 2018),
alpha-cellulose, holo-cellulose, and acid-insoluble-
lignin (Pan et al., 2017), anthocyanins (Dai et al.,
2016), phenols extraction from Cajanus cajan (Wei
et al., 2012) and olive cake, onion seed, tomato and
pear (agro-food industrial by-products (Fernández et
al., 2017), anthocyanins from Catharanthus roseus
(Dai et al., 2016), resveratrol from Morus alba
(Alishlah, Mun’in, and Jufri, 2019) and peanut (Chen
et al., 2018), and so on. However, the extraction of
total polyphenolic content from M. speciosa leaves
has not reported.
In the present study, the extraction of total
polyphenolic content (TPC) was performed by using
NADES with some combination type different and
combined with microwave-assisted extraction
(MAE). The study aims to determine the effect of
using natural deep eutectic solvent-based microwave-
assisted extraction (NADES-MAE) on total
polyphenol content (TPC) extraction from M.
speciosa leaves.
2 MATERIALS AND METHODS
2.1 Materials
The sample of M. speciosa leaf was obtained from
Melak, Kutai Barat, East Kalimantan, Indonesia and
were authenticated at Laboratory of Dendrology,
Faculty of Forestry, Universitas Mulawarman,
Samarinda, East Kalimantan, Indonesia. The sample
specimen was achieved at the Laboratory of
Pharmaceutical Research and Development
“FARMAKA TROPIS,” Faculty of Pharmacy,
Universitas Mulawarman, Samarinda, East
Kalimantan, Indonesia. Citric acid, lactic acid,
sucrose, glucose, malic acid, choline chloride,
sorbitol were purchased from CV. Chlorogreen,
Bandung, West Java, Indonesia. Gallic acid standard,
Folin-Ciocalteu reagent, and sodium carbonate were
purchased from Sigma-Aldrich, USA (via PT. Elo
Karsa Utama, Indonesia).
2.2 Extraction Process
2.2.1 Preparation of Natural Deep Eutectic
Solvent (NADES)
In this study, for screening of NADES was used
including citric acid–glucose (CAG), malic acid–
glucose (MAG), lactic acid–sucrose (LAS), and
choline chloride–sorbitol (CCS) with ratio of 4:1 g/g,
1:2 g/g, 1:1 g/g, and 1:2 g/g, respectively. The
NADES component is weighed according to each
ratio, then melted on a magnetic stirrer hotplate. Aqua
demineralization is added according to the number of
comparisons used. The mixture is stirred until
homogeneous. The solution is stored in a closed bottle
(Ahmad et al., 2018).
2.2.2 Extraction using NADES
A natural deep eutectic solvent-based microwave-
assisted extraction (NADES-MAE) was performed to
obtain total polyphenolic content (TPC) according to
some literature (Z. F. Wei et al., 2015; Z. Wei et al.,
2015; Dai et al., 2016; Ahmad et al., 2017b; Savi et
al., 2018). Briefly, the powder simplicial of M.
speciosa (5 gram) was extracted for 10 minutes (with
30% microwave power) using NADES-MAE method
which some combination types of NADES. The
sample residue and extract solution were separated
using the Buchner funnel. The extract was deposited
at a cold temperature and until ready to use. Whereas
extraction using ethanol solvents was carried out by
maceration. Samples are immersed in a solvent for 1
x 24 hours continuously, maceration is stopped when
the solvent has begun to clear. The extract solution
and the sample residue are separated using a
separating funnel, then evaporated to obtain a dry
extract.
2.3 Total Polyphenolic Content (TPC)
Determination
The TPC was evaluated by using spectrophotometer
UV-Vis method based on the literature (Bobo-García
et al., 2014; Do et al., 2014; Ahmad et al., 2015) with
Enhanced Extraction of Total Polyphenols Content from Mitragyna Speciosa (Korth.) Havil Leaves using the Natural Deep Eutectic
Solvent-based Microwave-assisted Extraction Method
73
slight modification. Briefly, the sample and standard
solution (1 mL) was added to 5 mL aqua
demineralization and 0.5 mL Folin-Ciocalteau
reagent, homogenized and allowed for 5 minutes.
Next, a 2 mL sodium carbonate solution was added,
homogenized, and incubated for 30 minutes. The
absorbance was measured using spectrophotometer
UV-Vis with 770 nm. The standard solution of gallic
acid (with a concentration of 12.5 ppm, 25 ppm, 50
ppm, 100 ppm, and 100 ppm, respectively) was used
to obtain the regression formula: Y = a + bX, where
X is concentration, and Y is absorbance.
3 RESULTS AND DISCUSSION
3.1 Extraction Process
The application of NADES to extract target
secondary metabolite compounds from natural
materials is expected to be an alternative solvent
option to be able to replace conventional organic
solvents. At this stage, it only focuses on the selection
of NADES combination types that refer to previous
studies (García et al., 2016; Fernández et al., 2017; Z.
F. Wei et al., 2015; Dai et al., 2016; Wang et al., 2018;
Ahmad et al., 2018; Alishlah, Mun’in, and Jufri,
2019; Yin-Leng and Suyin, 2019; Yuniarti, Saputri,
and Mun'im, 2019). The NADES combination types
were used in this study include citric acid–glucose
(CAG), malic acid–glucose (MAG), lactic acid–
sucrose (LAS), and choline chloride–sorbitol (CCS)
with ratio of 4:1 g/g, 1:2 g/g, 1:1 g/g, and 1:2 g/g,
respectively. While other factors such as extraction
time, solvent and sample ratios, microwave power,
and concentration of aqua demineralization were
carried out under constant conditions.
In this study, the TPC extraction was performed
by using the NADES-MAE method. The selection of
the extraction method is based on the effectiveness of
the use of the solvent, the extraction time, the cost
efficiency, and the stability of the target compound.
NADES-MAE was chosen because it is
environmentally friendly, safe, inexpensive, low
toxicity, and fast.
3.2 Total Polyphenolic Content (TPC)
The determination of the TPC was performed by
using spectrophotometer UV-VIS at 746 nm with
Folin-Ciocalteau reagent. The measurement results of
standard gallic acid shown in Table 1. According to
the results, it shows that the absorbance measurement
results at each concentration are outstanding namely
in the absorbance range of 0 up to 1 and following the
literature (Bobo-García et al., 2014; Ahmad et al.,
2017a).
Table 1: The absorbance results of the gallic acid standard.
Concentration
(ppm)
Absorbance Average
Absorbance
Standard
Deviation
12.5
0.024
0.025
0.025
0.024 0.0006
25
0.053
0.058
0.067
0.059 0.0071
50
0.092
0.106
0.107
0.101 0.0084
100
0.229
0.238
0.233
0.233 0.0045
200
0.430
0.445
0.446
0.440 0.0090
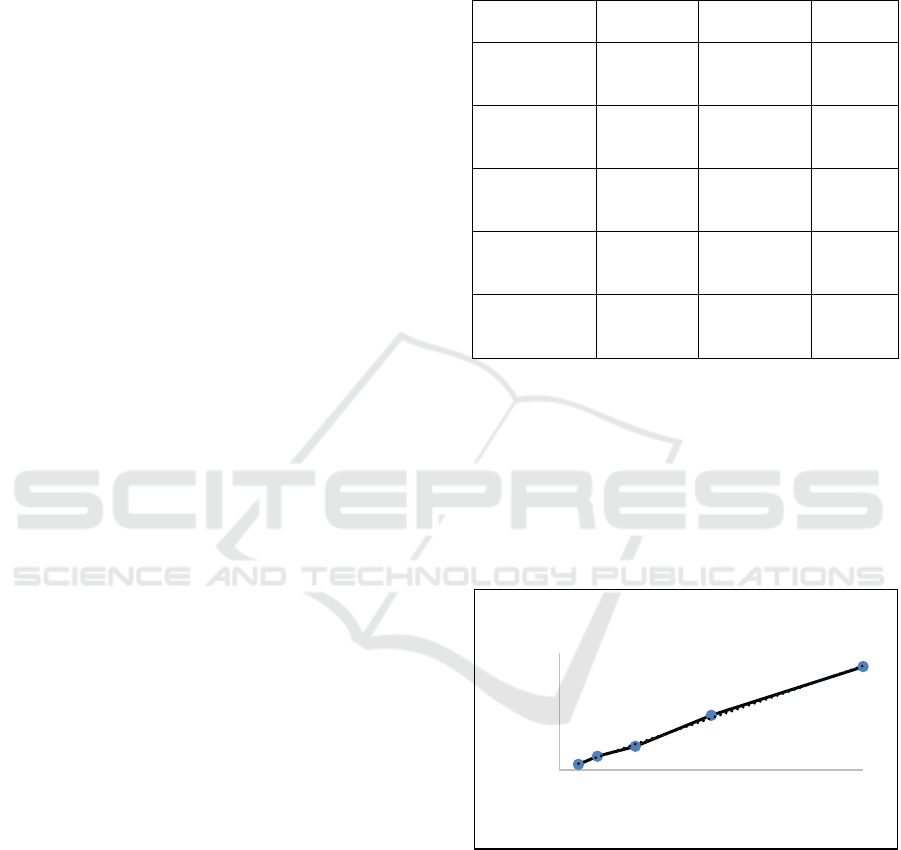
Based on the calculation results of the linear
regression analysis shown in Figure 1, the equation
obtained Y= 0.0022X-0.00095 with a correlation
coefficient (R2) of 0.998 (Figure 1). Where Y is
absorbance value, and X is the concentration of gallic
acid standard. The equation formula was used to
calculate the TPC from M. speciosa leaves by using
different NADES combination types compared
conventional organic solvent and extraction method.
Figure 1: Regression curve of gallic acid standard.
According to the absorbance measurements for
each extract (NADES combination type and ethanol),
different absorbances were obtained and were in the
absorbance range of 0 to 1 (at the concentration of the
diluted sample) shown in Table 2. The TPC was
calculated based on sample weight (mg GAE/g
sample).
y = 0.0022x-0.00095
R² = 0.9977
0
0,1
0,2
0,3
0,4
0,5
0 50 100 150 200
Absorbance
Concentration
Regression Curve
ICOH 2019 - 1st International Conference on Health
74
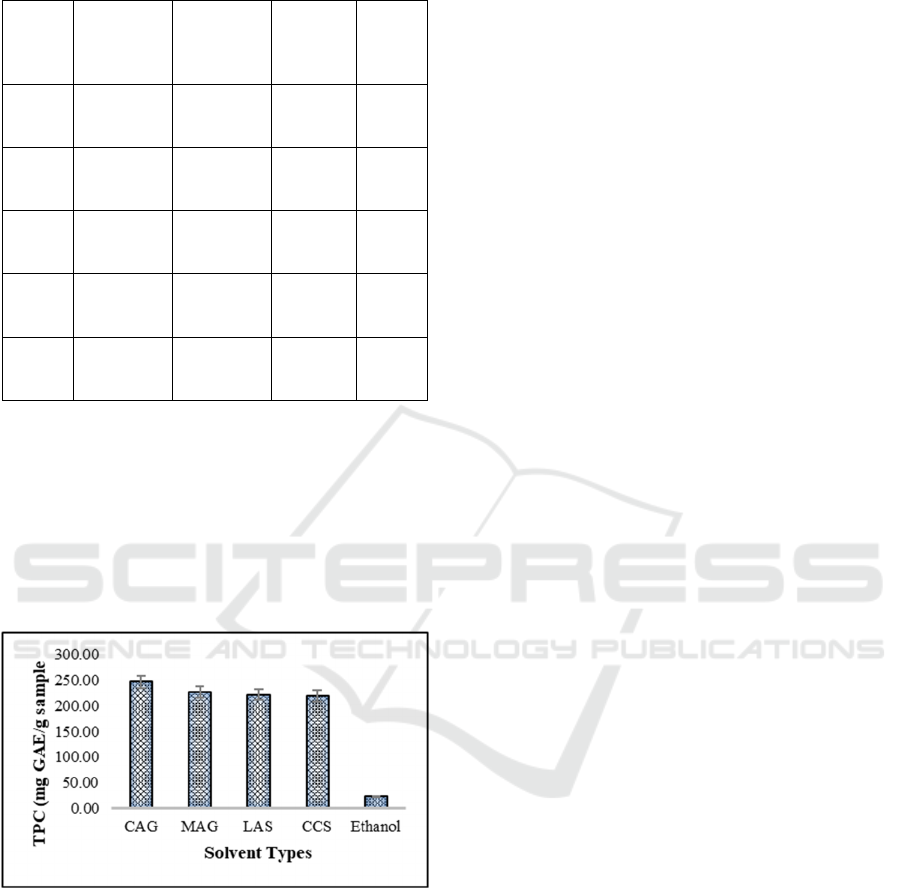
Table 2: Total polyphenolic content (TPC) of M. speciosa
leaves based on the solvent type used.
Solvent
Types
Absorbance
Average
Absorbance
Standard
Deviation
TPC
(mg
GAE/g
sam
p
le
)
CAG
0.929
0.845
0.853
0.875 0.0466 246.70
MAG
0.651
0.662
0.668
0.660 0.0084 227.33
LAS
0.850
0.861
0.921
0.877 0.0382 222.26
CCS
0.738
0.798
0.795
0.777 0.0338 219.02
Ethanol
0.560
0.526
0.528
0.538 0.0191 23.12
Based on the obtained TPC results (as can be seen
in Figure 2), shows that the NADES combination type
of CAG has a maximum yield of TPC (with a TPC
value of 246.70 mg GAE/g sample) compared to
other NADES combination types. But in general, it
shows that the use of the NADE-MAE method is
beneficial for extracting target secondary metabolites
(mainly TPC) compared to conventional organic
solvents.
Figure 2: Efficiency extraction of TPC from M. speciosa
leaves.
This research is the early step in developing an
extraction method to obtain target secondary
metabolites from M. speciosa leaves efficiently,
easily, quickly, and safely. Furthermore, optimization
of the NADES-MAE method will be carried out using
the response surface methodology, identification of
active secondary metabolites, and the scale-up
extraction based on NADES-MAE.
4 CONCLUSIONS
According to the above results, the use of NADES-
MAE method based on NADES combination type is
beneficial to obtain TPC value compared with the
other NADES combination type and conventional
extraction method. The highest TPC value of 246.7;
227.33; 222.26; and 219.02 mg GAE/g sample was
obtained by using citric acid-glucose (4:1 g/g); malic
acid-glucose (1:2 g/g); lactic acid-sucrose (1:1 g/g),
and choline chloride-sorbitol (1:2 g/g), respectively.
This result was new data for the next study based on
NADES-MAE methods efficiently, easily, quickly,
and safely.
ACKNOWLEDGMENTS
This research supported by the Ministry of Research,
Technology, and Higher Education, Republic of
Indonesia and Lembaga Penelitian dan Pengabdian
Kepada Masyarakat Universitas Mulawarman (LP2M
UNMUL) via a grant “Hibah Penelitian Dasar
Unggulan Perguruan Tinggi (PDUPT) 2019-2020.
REFERENCES
Ahmad, A., Husain, A., Mujeeb, M., Khan, S. A.,
Alhadrami, H. A. A., Bhandari, A. 2015. Quantification
of total phenol, flavonoid content and
pharmacognostical evaluation including HPTLC
fingerprinting for the standardization of Piper nigrum
Linn fruits. Asian Pacific Journal of Tropical
Biomedicine. 5(2), pp. 101–107.
Ahmad, I., Yanuar, A., Mulia, K., Mun'im, A. 2017a.
Extraction of polyphenolic content from Peperomia
pellucida (L) Kunth herb with 1-ethyl-3-
methylimidazolium bromide as a green solvent. Indian
Journal of Pharmaceutical Sciences, 79(6), pp. 1013–
1017.
Ahmad, I., Yanuar, A., Mulia, K., Mun'im, A. 2017b.
Optimization of ionic liquid-based microwave-assisted
extraction of polyphenolic content from Peperomia
pellucida (L) Kunth using response surface
methodology. Asian Pacific Journal of Tropical
Biomedicine, 7(7), pp. 660–665.
Ahmad, I., Pertiwi, A.S., Kembaren, Y.H., Rahman, A.,
Mun'im, A. 2018. Application of natural deep eutectic
solvent-based ultrasonic assisted extraction of total
polyphenolic and caffeine content from Coffe Beans
(Coffea Beans L.) for instant food products. Journal of
Applied Pharmaceutical Science, 8(8), pp. 138–143.
Alishlah, T., Mun’in, A., Jufri, M. 2019. Optimization of
urea-glycerin based NADES-UAE for oxyresveratrol
extraction from Morus alba roots for preparation of
Enhanced Extraction of Total Polyphenols Content from Mitragyna Speciosa (Korth.) Havil Leaves using the Natural Deep Eutectic
Solvent-based Microwave-assisted Extraction Method
75

skin whitening lotion. Journal of Young Pharmacist,
11(2), pp. 155–160.
Bobo-García, G., Davidov-Pardo, G., Arroqui, C., Marin-
Arroyo, M. R., Navarro, M., Virseda, P. 2014. Intra-
laboratory validation of microplate methods for total
phenolic content and antioxidant activity on
polyphenolic extracts, and comparison with
conventional spectrophotometric methods. Journal of
Science Food and Agricultur, 95(1), pp. 204–209.
Brown, P. N., Lund, J. A., Murch, S. J. 2017. A botanical,
phytochemical and ethnomedicinal review of the genus
Mitragyna korth: Implications for products sold as
kratom. Journal of Ethnopharmacology, 202, pp. 302–
325.
Chen, J., Jiang, X., Yang, Guolong, Bi, Y., Liu, W., 2018.
Green and efficient extraction of resveratrol from
peanut roots using deep eutectic solvents. Journal of
Chemistry, 2018, pp. 1–10.
Chittrakarn, S., Penjamras, P., Keawpradub, N. 2012.
Quantitative analysis of mitragynine, codeine, caffeine,
chlorpheniramine and phenylephrine in a kratom
(Mitragyna speciosa Korth.) cocktail using high-
performance liquid chromatography. Forensic Science
International, 217(1–3), pp. 81–86.
Dai, Y., Rozema, E., Verpoorte, R., Choi, Y.H. 2016.
Application of natural deep eutectic solvents to the
extraction of anthocyanins from Catharanthus roseus
with high extractability and stability replacing
conventional organic solvents. Journal of
Chromatography A, 1434, pp. 50–56.
Do, Q. D., Angkawijaya, A. E., Tran-Nguyen, P. L., Huynh,
L. H., Soetaredjo, F. E., Ismadji, S. 2014. Effect of
extraction solvent on total phenol content, total
flavonoid content, and antioxidant activity of
Limnophila aromatica. Journal of Food and Drug
Analysis, 22(3), pp. 296–302.
Fernández, M. Á., Espino, M., Gomez, F. J. V., Silva, M.
F. 2017. Novel approaches mediated by tailor-made
green solvents for the extraction of phenolic
compounds from agro-food industrial by-products.
Food Chemistry, 239, pp. 671–678.
García, A., Rodriguez-Juan, E., Rodriguez-Gutierrez, G.,
Rios, J. J., Fernandez-Bolanos, J. 2016. Extraction of
phenolic compounds from virgin olive oil by deep
eutectic solvents (DESs). Food Chemistry, 197, pp.
554–561.
Gomez, F. J. V., Espino, M. 2018. A greener approach to
prepare natural deep eutectic solvents. Analytical
Chemistry, 3, pp. 6122–6125.
Hassan, Z., Muzaimi, M., Navaratnam, V., Yusoff, N. H.
M., Suhaimi, F. W., Vadivelu, R., Vicknasingam, B. K.,
Amato, D., von Horsten, S., Ismail, N. I. W., Jayabalan,
N., Hazim, A. I., Mansor, S. M., Muller, C. P. 2013.
From Kratom to mitragynine and its derivates:
Physiological and behavioural effets related to use,
abuse, and addiction. Neuroscience and Biobehavioral
Reviews, 37, pp. 138-151.
Henningfield, J. E., Fant, R. V., Wang, D. W. 2018. The
abuse potential of kratom according the 8 factors of the
controlled substances act : implications for regulation
and research. Psychopharmacology, pp. 573–589.
Horie, S., Koyama, F, Takayama, H., Ishikawa, H., Aimi,
N., Ponglux, D., Matsumoto, K., Murayama, T. 2005.
Indole alkaloids of a Thai medicinal herb, Mitragyna
speciosa, that has opioid agonistic effect in guinea-pig
ileum. Planta Medica, 71(3), pp. 231–236.
Luliana, S., Robiyanti, Islamy, M. R. 2018. Antinociceptive
activity of dichloromethane fraction of Kratom leaves
(Mitragyna speciosa Korth.) by oral route in male swiss
mice. Pharmaceutical Sciences and Research, 5(2), 58-
64.
Mossadeq, W. M. S., Sulaiman, M. R., Tengku Mohamad,
T. A., Chiong, H. S., Zakaria, Z. A., Jabit, M. L.,
Baharuldin, M. T. H., Israf, D. A. 2009. Anti-
inflamatory and antinociceptive effects of Mitragyna
speciosa Korth methanolic extract. Medical Principles
and Practice, 18, 378-384.
Pan, M., Zhao, G., Ding, C., Wu, B., Lian, Z., Lin, H. 2017.
Physicochemical transformation of rice straw after
pretreatment with a deep eutectic solvent of choline
chloride/urea. Carbohydrate Polymers, 176(5), pp.
307–314.
Parthasarathy, S., Azizi, J., Ramanathan, S., Ismail, S.,
Sasidharan, S., Mohd. Said, M.I., Mansor, S.M. 2009.
Evaluation of antioxidant and antibacterial activities of
aqueous, methanolic and alkaloid extract from
Mitragyna speciosa (Rubiaceae Family) leaves,
Molecules, 14, 3964-3974
Saingam, D., Assanangkornchai, S., Geater, A. F., Balthip,
Q. 2013. Pattern and consequences of krathom
(Mitragyna speciosa Korth.) use among male villagers
in southern Thailand : A qualitative study. International
Journal of Drug Policy, 24(4), pp. 351–358.
Savi, L. K., Dias, M. C. G. C., Carpine, D., Waszcynskyj,
N., Ribani, R. H., Haminiuk, C. W. I. 2018. Natural
deep eutectic solvents (NADES) based on citric acid
and sucrose as a potential green technology : a
comprehensive study of water inclusion and its effect
on thermal, physical and rheological properties.
International Journal of Food Science and Technology,
54(3), pp. 898–907.
Takayama, H. 2004. Chemistry and pharmacology of
analgesic indole alkaloids from the rubiaceous plant,
Mitragyna speciosa. Chemical & pharmaceutical
bulletin, 52(8), pp. 916–928.
Tohar, N., Shilpi, J. A., Sivasothy, Y., Ahmad, S., Awang,
K. 2019. Chemical constituents and nitric oxide
inhibitory activity of supercritical carbon dioxide
extracts from Mitragyna speciosa leaves. Arabian
Journal of Chemistry, 12(3) pp. 350-359.
Wang, H., Ma, X., Cheng, Q, Xi, X., Zhang, L. 2018. Deep
eutectic solvent-based microwave-assisted extraction
of baicalin from Scutellaria baicalensis Georgi.
Journal of Chemistry
, 2018, pp. 1–10.
Wei, W., Fu, Y.J., Zu, Y.G., Wang, W., Luo, M., Zhao, C.J.,
Li, C.Y., Zhang, L., Wei, Z.F. 2012. Ionic liquid-based
microwave-assisted extraction for the determination of
flavonoid glycosides in pigeon pea leaves by high-
performance liquid chromatography-diode array
ICOH 2019 - 1st International Conference on Health
76

detector with pentafluorophenyl column. Journal of
Separation Science, 35(21), pp. 2875–2883.
Wei, Z., Qi, X., Li, T., Luo, M., Wang, W., Zu, Y., Fu, Y.
2015. Application of natural deep eutectic solvents for
extraction and determination of phenolics in Cajanus
cajan leaves by ultra performance liquid
chromatography. Separation and Purification
Technology, 149, pp. 237–244.
Wei, Z. F., Wang, X. Q., Xiao, P., Wei, W., Zhao, C. J., Zu,
Y. G., Fu, Y. J. 2015. Fast and green extraction and
separation of main bioactive flavonoids from Radix
Scutellariae. Industrial Crops and Products, 63, pp.
175–181.
Yin-Leng, K., Suyin, G. 2019. Natural deep eutectic solvent
(NADES) as a greener alternative for the extraction of
hydrophilic (Polar) and Lipophilic (Non-Polar)
phytonutrients. Key Engineering Materials, 797, pp.
20–28.
Yuniarti, E., Saputri, F. C., Mun'im, A. 2019. Application
of the natural deep eutectic solvent choline chloride-
sorbitol to extract chlorogenic acid and caffeine from
green coffee beans (Coffea canephora). Journal of
Applied Pharmaceutical Science, 9(03), pp. 82–90.
Enhanced Extraction of Total Polyphenols Content from Mitragyna Speciosa (Korth.) Havil Leaves using the Natural Deep Eutectic
Solvent-based Microwave-assisted Extraction Method
77
