
Determination of Total Flavonoid Levels in Packaged Tea Bags
Combination of Dayak Onion and Beet Root with UV Visible
Spectrophotometric Method
Rahma Yulia
1
, Adewirli Putra
2
, Yulinda Rahmi
3
Mohammad Natsir University, Bukittinggi, Indonesia
Keywords: Total Flavonoid; Dayak Onion; Beet Root; Tea Bag; Antioxidants.
Abstract: The utilization of natural resources that contain antioxidants is in high demand by the community to prevent
degenerative diseases such as heart disease, diabetes, stroke and cancer. Processing of Dayak onion and beet
root in the form of herbal tea has great potential to be used as a functional drink because both of these plants
contain flavonoids which have antioxidant properties. In 5 tea formulas made by comparing the composition
of Dayak onions and beet root, a preference test was conducted on several panelists related to color, aroma
and taste and obtained 3 formulas favored by the panelists are formula1, 3 and 4. the purpose of this study
was to determine the total flavonoid levels of each of the teabag formulas favored by panelists using the UV
Visible spectroprotometry method with a quercetin comparison. From the research conducted obtained linear
regression equation data y = 0.0364x + 0.0028 with r = 0.997. Total flavonoid levels for each teabag
combination of dayak onion and beet root for formula 1; formula 3; formula 4 is 0.9309%; 0.975% and
1.841%, where the highest total flavonoid content is formula 4, which is 1, 841%.
1 INTRODUCTION
Bukittinggi is one of the tourism city in West
Sumatra, which is famous for its various culinary
delights and delicious flavors. Many culinary
preparations using spices and coconut milk as the
basic ingredients. The pattern of life of the people of
Bukittinggi who often consume foods containing
coconut milk and fatty foods causes the people of
Bukittinggi to have great potential to get a
degenerative diseases such as heart disease, diabetes
and stroke. The high price of modern medicines
encourages consumers to try other alternatives by
using the trend back to nature to maintain their health.
one of the natural product that have the potential to
overcome these degenerative diseases is plants that
contain antioxidant compounds.
Utilization of natural resources that contain
antioxidants is in high demand by the community to
prevent degenerative diseases. Antioxidants are
substances that at low concentrations can prevent or
slow down the oxidation process by binding to free
radicals and highly reactive molecules so that cell
damage can be inhibited. This compound has a small
molecular weight but is able to inactivate the
development of oxidation reactions by preventing the
formation of radicals (Dimitrios, 2006). Antioxidants
are generally present naturally in an important role for
the protection of body health. This compound can
prevent oxidative damage and reduce the risk of
disease.
Among the plants that contain lots of antioxidants
are Dayak onions (Eleutherine Palmifolia) and
beetroot (Beta vulgaris). Both of these plants have
identical colors that are purplish red which contain
bioactive compounds such as phenols, flavonoids,
taanin, glycosides, steroids and alkaloids (Claudea,
2013, Puspadewi, 2013). purplish red color in both
plants is caused by anthocyanin pigment content.
Anthocyanins are a group of pigments that cause
reddish color, located in water soluble cells in water
(Jaya 2013). Besides functioning as an antioxidant,
anthocyanin has other uses including as a natural
indicator, and as a coloring agent textile and food
industries (Hendrawan 2011). Several studies have
been carried out using anthocyanin pigments from
beetroot and dayak onions for natural dyes in food or
natural dyes for cosmetics such as lipsticks, but no
studies have been reported regarding the manufacture
of functional drinks from these two plants.
Diversification of natural products that are
traditionally processed can be done one of them by
356
Yulia, R., Putra, A. and Rahmi, Y.
Determination of Total Flavonoid Levels in Packaged Tea Bags Combination of Dayak Onion and Beet Root with UV Visible Spectrophotometric Method.
DOI: 10.5220/0009515103560363
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 356-363
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
making herbal tea drinks that are packaged in dyed
containers like tea drinks in general. Herbal Tea is
one of the tea beverage products from herbal plants
that has properties in helping the treatment of an
illness or as a body refreshing drink (Hambali et al,
2005, Mun’in, 2008). This product is a form of
change in health products.
Processing of Dayak onions and beetroot in the
form of herbal tea has great potential to become a
functional beverage, because it is related to its natural
antioxidant content, both plants can be utilized to
protect the body from free radical attack which results
in degenerative diseases.
Research conducted by Yulia et al, 2019,
regarding the formulation of tea bags with a
combination of dayak onions and beetroot with five
formulas containing different composition of dayak
onions and beetroot. After the hedonic test related to
the color, smell, taste and shape of the 5 formulas and
obtained three formulas favored by the panelists
namely formulas 3, 1 and 4.
Three formulas are preferably carried out
quantitative analysis of total flavonoid levels to find
out how much flavonoid levels are measured in the
tea packaging combination of dayak onions and
beetroot. So that it can be chosen among the three
formulas that have the highest flavonoid content so
that it can be developed into a health functional
beverage product. Determination of total flavonoid
levels was carried out by the method of chang et al
2002 using UV Visible Spectrophotometry.
2 MANUSCRIPT PREPARATION
2.1 Material
The Material used include five packaged teabag
formulas that have been studied by Yulia, et. al
(2019), Ethanol 95%, Methanol, Pure Quercetin,
Aquadest, 10% AlCl3 buffer solution, Sodium
Acetate buffer solution, Ethyl acetate, 4N HCl
solution.
2.2 Tool
The tools used in this study include a funnel, beaker
glass (pyrex), erlenmeyer (pyrex), stir bar, measuring
flask (pyrex), volume pipette (pyrex), test tube,
measuring cup (pyrex), UV-Vis spectrophotometer
1800 (pyrex) Shimadzu), pH meter.
2.3 Research Stages
There are three stages of research in this study the first
is the preparation and formulation of tea bags (Yulia,
et.al. 2019). The second stage was the packaging of
tea bags using 95% ethanol and the preparation of
standardized quercetin solutions and test solutions,
and the third step was the determination of total
Flavonoid and Anthocyanin levels using a UV-Vis
spectrophotometer.
2.3.1 Formula Teabag Combination of Beet
root-Dayak Onion
There are five Dayak onion-beetroot teabag formulas
made by comparison of different combination
ingredients (Yulia et al. 2019) as shown in Table 1.
Table 1. Beetroot - Dayak Onions Teabag Formulas
Formula
Code
Beet
Root
(gram)
Dayak
Onion
(gram)
Stevia
Leaves
(gram)
F1
1,5
0,5
0,15
F2
1
1
0,15
F3
0,75
1,25
0,15
F4
0,5
1,5
0,15
F5
0,25
1,75
0,15
2.3.2 Manufacture of Extractions, Standard
Solutions and Test Solutions
Extraction
Weigh each sample of tea bags as much as 5 grams.
Enter each sample that has been weighed into each
100 ml measuring flask. Add 100 ml of 95% ethanol,
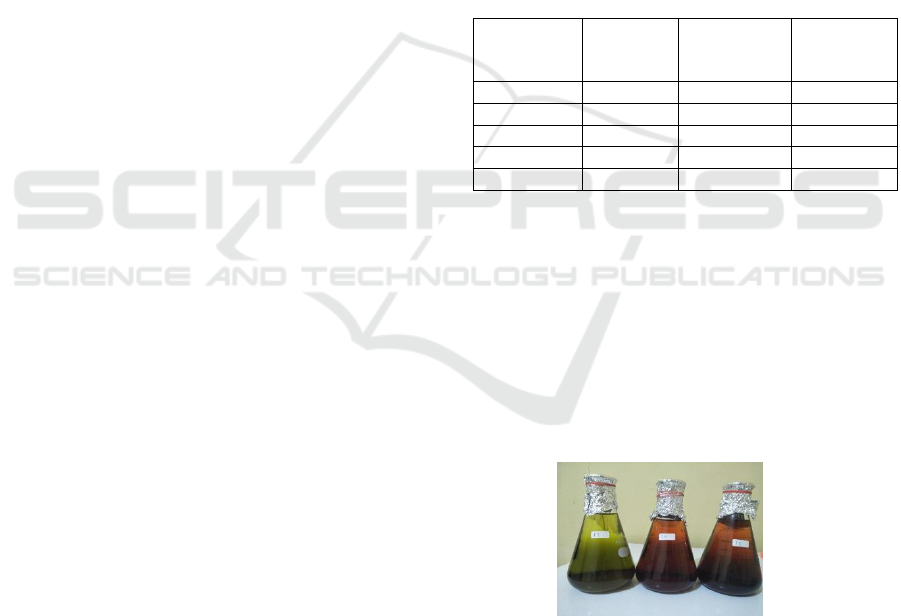
cover. Figure 1 is Maceration for 24 hours. The first
6 hours shake it many times. And leave it for 18
hours. Then filter the results of maceration.
Figure 1: maceration process of sample.
Quercetin Standard Solution
Pure quercetin weighed as much as 25 mg, dissolve
with methanol in a 100 ml volumetric flask to mark
the mark. This result is used as a standard solution.
The standard solution is then diluted with methanol in
6 different concentrations (Figure 2). Each
concentration was pipetted 2 ml, then 0.1 ml AlCl
3
Determination of Total Flavonoid Levels in Packaged Tea Bags Combination of Dayak Onion and Beet Root with UV Visible
Spectrophotometric Method
357

10% reagent was added, 0.1 ml sodium acetate, and
2.8 ml Aquadest, homogeneous and incubated for 30
minutes at room temperature. The absorbance was
measured on a UV-Vis 415 nm spectrophotometer
using blank solution without quercetin and AlCl
3
.
Figure 2. Quercetin Standard Solution
Sample Solutions
The extract that was made was weighed as much as 1
gram, then hydrolyzed with 4 ml HCl as much as 2 ml
for 30 minutes in a measuring flask. The solution is
then filtered and concentrated. Then the extract was
filtered with 15 ml of ethyl acetate, 3 times, the ethyl
acetate fraction was collected and concentrated. The
results of the ethyl acetate extract were put in a 25 ml
measuring flask and dissolved with methanol to the
limit mark (test solution). Do the same for the other 2
samples.
2.3.3 Determination of Total Flavonoid
Levels and Determination of
Anthocyanin Levels in Five Formulas
Was Carried out by the Chang Method
Determination of Total Flavonoid Levels
The test solution was pipetted as much as 0.5 ml, then
dissolved with 1.5 ml of methanol in the test tube,
then add a reagent consisting of 0.1 ml of 10% AlCl
3
,
0.1 ml of Sodium Acetate and 2.8 ml of distilled
water, homogeneous and incubation for 30 minutes at
room temperature. The absorption solution was
measured on a UV-Vis 415 nm spectrophotometer
using blank solution without the addition of AlCl
3
replaced with distilled water. Measurements were
made three times, levels were calculated as averages.
The total flavonoid content is expressed by
comparison equivalents of quercetin. Do the same
thing in the other 4 samples.
Determination of Anthocyanin Levels of Dayak
Onions – Beet root teabag
Determination of anthocyanin levels was done by
UV-Vis Spectrophotometer. The test solution was
made 5 grams of a mixture of tea combination of
dayak onions and beetroot extracted with 250 ml of
water for 5 minutes and 10 minutes. The test solution
was taken 1 ml, then two samples were measured
against 5 ml of pH solution 1.0 (buffer solution of
Potassium Chloride) and 4.5 (buffer solution of
Sodium Acetate). Furthermore, it was analyzed with
a UV-Vis spectrophotometer at a wavelength of 520-
700 nm.
3 RESULTS AND DISCUSSION
Beetroot is the taproot portion of a beet
plant, usually known in North America as the beet,
and also known as the table beet, garden beet, sugar
beet, red beet, dinner beet or golden beet. It is one of
several cultivated varieties of Beta vulgaris grown
for their edible taproots and leaves (called beet
greens), they have been classified as B.
vulgaris subsp. vulgaris 'Conditiva' Group. Besides
being used as a food, beets have uses as a food
colouring and as a medicinal plant. Many beet
products are made from other Beta vulgaris varieties,
particularly sugar beet.
In preliminary research, beetroot juice
reduced blood pressure in hypertensive
people. Tentative evidence has found that dietary
nitrate supplementation, such as from beets and other
vegetables, results in a small improvement in
endurance exercise performance. The red colour
compound betanin is not broken down in the body,
and in higher concentrations may temporarily cause
urine or stools to assume a reddish colour, in the case
of urine a condition called beeturia.
Although
harmless, this effect may cause initial concern due to
the visual similarity to what appears to be blood in the
stool, hematochezia (blood passing through the anus,
usually in or with stool) or hematuria (blood in the
urine). Nitrosamine formation in beet juice can
reliably be prevented by adding ascorbic acid.
Dayak onions are small bulbs that produce
several long, ribbon-like leaf stalks with a single
flowering stem. The smooth, dark red bulbs are
shaped like rounded diamonds with small, wispy
brown roots extending from the ends.
Research on Dayak onions has been carried out,
including plant bulbs of the genus Eleutherine
(Eleutherine bulbosa and Eleutherine Americana),
which are known to contain secondary metabolites of
the naphthoquinone group (elecanacin, eleutherin,
eleuthero, eleuthero). Onion Dayak has anti cancer
and anti oxidants, which are usually found in vacuole
cells in the form of glycosides. Some studies also
state the content of the active compounds in Dayak
onions is extensive, so it is very reasonable for
various properties. These compounds include
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
358

alkaloids, steroids, glycosides, flavonoids, phenolics,
tannins, and saponins. One of these compounds,
namely flavonoids, can be efficient as anticancer,
antiviral, anti inflammatory, reduce the risk of
cardiovascular disease, and free radical catchers.
According to D. Lestari et all, 2018, dayak
onion/tiwai onion contain secondary metabolites such
as alkaloids, flavonoids, tannins, and quinones. These
compounds are known to have a very broad biological
activity including antioxidants and anticancer.
Flavonoids are known to be good antioxidants
because they have at least two hydroxyl groups in
ortho positions and para which can capture free
radicals by freeing hydrogen atoms from their
hydroxyl groups. Flavonoids as antioxidants have a
higher potential as anticancer drugs than vitamins and
minerals. Flavonoid compounds can prevent the
reaction of carcinogen molecules joining cell DNA so
as to prevent cell DNA damage; the bioactive
components of flavonoids can prevent the initial
process of cancer cell formation. Flavonoids can
stimulate the regenaration process of mutated DNA
cell so that the cells become normal again. In
addition, quinone compounds have also been reported
to have antioxidant activity. It is likely that quinone
originates from the oxidation of the corresponding
phenols namely catechol to form ortho-quinones and
quinol producing para-quinones.
Beetroot and dayak onions in this research
obtained from palano stone plots in Agam Regency,
West Sumatra Province can be seen in Figures 1 and
2. Beetroot are used which have ± 10 weeks old.
Dayak onions used are ± 4 month old, onions or plants
have flowered, this is because the quality of the
Dayak onions is in an optimal state. Simplicia is
cleaned with running water to remove impurities
(Puspadewi, 2013). After the simplicia is selected
and cleaned, then thinly sliced to be dried using an
oven at 45 ± 2oC until perfect drying is obtained,
because the simplicia cannot be heated at high
temperatures because it will cause damage or loss of
secondary metabolites. This drying process intended
to reduce water content contained in the sample, so it
can prevent spoilage by bacteria. After the dried
simplicia is then made into powder using a blender,
the powder is weighed for each formula and packaged
into a tea bag.
Figure 3: Dayak Onion Figure 4: Beetroot
The packaging of tea bags is carried out with five
different formulations namely F1, F2, F3, F4 and F5
formulas. The results of the tea bag can be seen in
Figure 5.
Figure 5: Forms of Steeping five combination teabag
formula (Yulia,et.al.2019).
This Dayak onion-beetroot combination teabag is
one of the more practical forms of preparation,
preferred by the public and has antioxidant properties,
because each of the simplicia that has been previously
studied (Melisa, 2015 and Puspadewi, 2013) has a
compound content. Compounds that act as
antioxidants in simplicia include Flavonoids and
Anthocyanins. To determine the content of these
compounds in this teabag, the flavonoid content was
tested by UV-Vis spectrophotometer by Chang
(2002). Before conducting the test for determining the
level of flavonids carried out on this teabag extract
based on the Chang method. The extraction process is
aimed at take chemical compounds contained in the
sample. The principle of extraction is based on
displacement the mass of the components of the
substance dissolved into the solvent resulting in
displacement in the interface layer and diffuses into
the solvent (Harborne, J.B 1987).
To get chemical compounds that are the desired
extraction method is used is a method of extracting
nutritious substances or substances active parts of the
plant by using solvents appropriate (Yuliani &
Satuhu, 2012). The extraction method used on this
research is maceration, because of this method
simpler, easier and without heating. Because heating
can make flavonoid levels reduced. The calibration
curve with a standard solution of Quercetin which is
made with 6 concentrations shown in Table 2.
Determination of Total Flavonoid Levels in Packaged Tea Bags Combination of Dayak Onion and Beet Root with UV Visible
Spectrophotometric Method
359
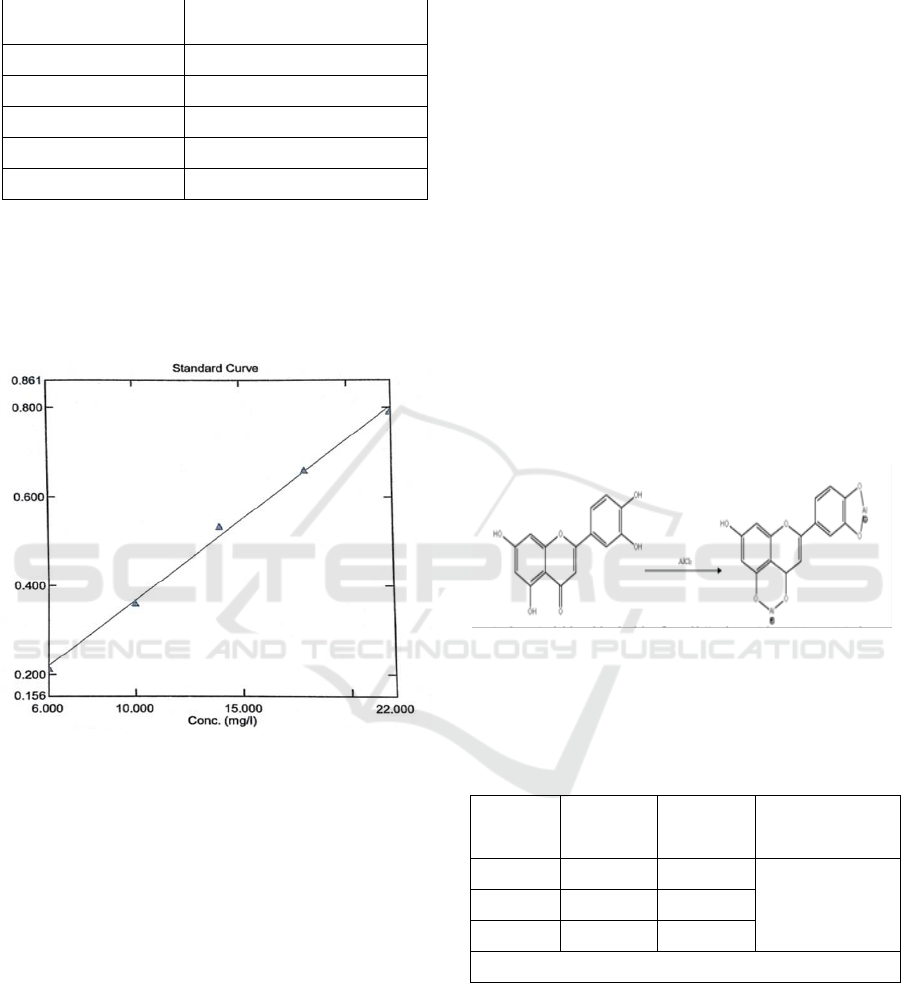
Table 2. Concentrations of quercetin standard solutions on
calibration curves
Concentrations
(ppm)
Absorbant (A)
6
0,215
10
0,360
14
0,533
18
0,659
22
0,792
Quersetin used (Figure 6) as a standard solution
because quercetin is flavonoid flavonol group which
has a group keto at C-4 and has a hydroxyl group on
neighboring C-3 or C-5 atoms from flavones and
flavonols (Azizah and Faramayuda 2014)
Figure 6: Quercetin calibration curve
The calibration curve obtained is y = 0.0364x +
0.0028, with r = 0.997. A value of r close to 1
indicates a linear calibration curve and there is a
relationship between the concentration of quercetin
solution and the absorption value, Azizah (2014).
This method was chosen because it is easier and
simpler, faster, economical, and is known to be more
specific to the flavonoid and flavonol groups.
Aluminum (III) chloride reagents are used to form
acid-resistant complexes with C-4 ketone groups and
C-3 or C-5 hydroxyl groups in flavones and flavonols,
and form acid-resistant complexes with ortho-
hydroxy groups in the ring A or B in flavonoids
(Chang et al., 2002 & Humadi,Istudor,2008). In the
measurement of total flavonoid compounds, the
sample solution is added AlCl
3
which can form
complexes, resulting in a shift in wavelength towards
the visible which is indicated by the solution
producing a more yellow color. And the addition of
potassium acetate which aims to maintain
wavelengths in visible areas (Chang et al, 2002). The
addition of aluminum chloride aims to form
complexes with quercetin (Indrayani, 2008). The
incubation treatment for 1 hour before the
measurement is intended so that the reaction runs
perfectly, so that the resulting color intensity is more
maximal (Azizah and Faramayuda 2014). Total
flavonoid levels are calculated as equivalence of
quercetin raw materials (Humadi, Istudor, 2008).
In general, the determination of total flavonoid
levels in a plant sample is based on the formation of
aluminum complex compounds (Al-Flavonoids), in
the form of a yellow solution. The addition of acetate
salts to the determination of flavonoid levels is
intended to produce shifts and stronger peak
absorbance intensities (Pekal & Pyrzynska, 2014).
Al-Flavonoid chelate complexes are formed in ketone
groups and hydroxyl groups of flavonoids
(Sepahpour, Selamat, Manap, & Razis, 2018), the
reactions that occur can be seen in Figure 7.
Figure 7: Chemical reaction of Flavonoid with AlCl
3
From this method, the results of determining the total
Flavonoid levels are obtained in Table 3.
Table 3: Determination of total flavonoid levels of teabag
combination with beetroot and dayak onions.
Extract
weight
Absorbant
at λ 436,5
nm (y)
Total
flavonoid
levels (%)
Average total
flavonoid levels
(%)
0,175 g
2.372
0,9309
1,249
0,146 g
2.072
0,975
0,105 g
2,815
1,841
the range of total flavonoid levels = 0.9307-1.841%
Total flavonoid levels obtained for formula 1
teabag combination of beetroot and dayak onions is
0.9303%, for formula 3 is 0, 975% and total flavonoid
level for formula 4 is 1.841% with an average total
flavonoid level is 1, 249%
Determination of anthocyanin levels was carried
out on the three best formulas from the results of the
study (Yulia, et.al.2019). Determination of the levels
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
360

(Figure 8) of anthocyanin extracted in the same way
according to the way the tea is served, which is
brewed for a few minutes in accordance with the way
tea is served in general, in the experiment 5 minutes
and 10 minutes are chosen to see the levels of
anthocyanin with different extraction times. Figure 8
explains the sample solution for determining
anthocyanin levels.
Figure 8: Sample solution for determination anthocyanin
levels.
Anthocyanins are included in the class flavonoid
compounds, constitute a group the biggest natural
pigment in plants which dissolve in ak which is
responsible for give color to flowers, fruit and
veegetables. Anthocyanins can be beneficial for
health as a source of antioxidants. This is due to the
compound This polyphenolic is a derived glycoside
polyhydroxy and polymethoxy from, 2-
phenilbenzopirilium or flavilium salt.
Anthocyanins are in several equilibrium form.
Kinbtika study and thermodynamics that are studied
in general accepting the transformation of differences
(proton transfer, isomerization and tautomerization)
flavilium cation from simple anthocyanin under
various pH conditions. In acidic solution stronger
(under pH 2) more flavilium cations dominant and
provides anthocyanin solutions the Red one.
The anthocyanin stability is not only influenced
by the heating temperature in the processing only, but
also influenced by intrinsic factors and extrinsic in the
product, such as pH, temperature storage, chemical
structure and concentration existing anthocyanins, the
presence of light, oxygen, enzymes, proteins, and
metal ions. to know the stability of anthocyanin is
needed initial data on anthocyanin levels from the
starting material contain these substances.
Determination of anthocyanin is done with the pH
difference method of pH 1.0 and pH 4.5. At pH 1.0
anthocyanin is formed oxonium compound. The
increasingly situation especially if it is nearing pH I
will cause more pigment anthocyanins are in the form
of cations colored flavilium or oxonium and
absorbance measurement will show an increasingly
large amount of anthocyanin. At pH 4.5, it is on weak
acids Flavilium cations change into more forms stable
colorless hemiketal and shape calchont (Figure 9).
Orange-purple flavilium cation colorless hemiketal for
pH 1.0 pH 4.5
Figure 9: Flavilium cation structure and hemiketal
shape R=H Glicocyde substitutens.
Measurement of anthocyanin levels using UV-
Vis Spectrophotometry, as previous studies
anthocyanin levels were calculated using the general
equation :
Concentracion (mg/ml) = A x MW x FD x 1000
/ (ϵ x l)
Information :
A = absorbance
= A 535.2 (pH 1.0) - A535.2 (pH 4.5)
MW = molecular weight = 433.2 g / mol
FD = dilution factor
ϵ = molar absorption = 31600 L / cm mol
l = cuvette width (1 cm)
Determination of anthocyanin levels was carried
out three times (Triplo) at the maximum wavelength
obtained which is 518 nm, the maximum wavelength
is then used to measure the absorption of the
calibration curve and extract sample (Azizah, 2014).
It is known that the main wavelength of anthocyanin
is in the range of 475-560 nm (Harborne, 1987). From
the results of determining the level of anthocyanin
obtained it can be said that the brewing of tea for 5
minutes and 10 minutes is almost the same so that the
brewing time can be recommended for only 5
minutes. Anthocyanin levels were determined using
UV-Vis spectrophotometry, based on the ability of
anthocyanins to produce colors at pH 1.0 (buffered
potassium chloride solution) and pH 4.5 (sodium
acetate buffer solution). The measurement data using
UV-Vis spectrophotometer is shown in Table 4.
Determination of Total Flavonoid Levels in Packaged Tea Bags Combination of Dayak Onion and Beet Root with UV Visible
Spectrophotometric Method
361
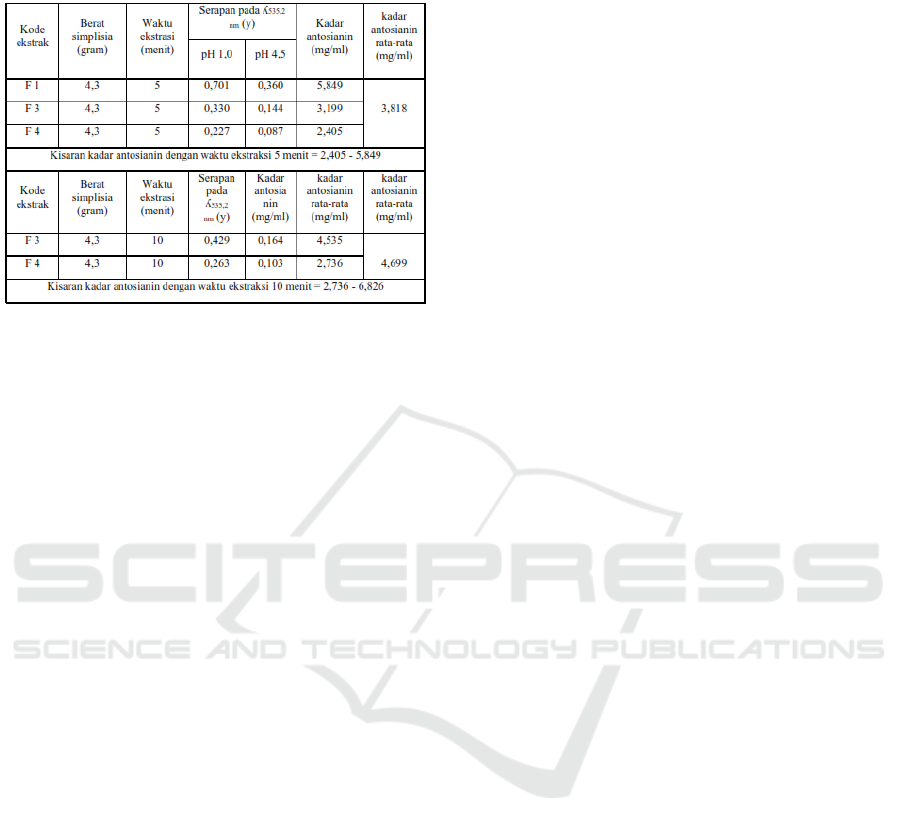
Table 4. Determination of anthocyanin levels of teabag
extract combined with beetroot and dayak onions
This characteristic depends on the transformation
of the chromophore structure. The color of the
anthocyanin ion stands out at pH 1.0. While the
colorless hemiketal structure at pH 4.5 (Herrera,
2004)
4 CONCLUSIONS
Determination of total flavonoid and anthocyanin
levels. The results obtained for total flavonoid levels
were tested on the three best formulas obtained in
previous studies (Yulia, et.al. 2019), namely the F1,
F3 and F4 formulas respectively were obtained
0.9309%, 0.975%, 1.841%, Whereas for anthocyanin
levels during 5 minutes, 5,849 mg / ml, 3,199 mg /
ml, 2,405 mg / ml were obtained. And for 10 minutes,
6.826 mg / ml, 4.535 mg / ml, 2.736 mg / ml were
obtained.
ACKNOWLEDGEMENTS
Acknowledgement to the Ministry of Research and
Technology of Higher Education for funding this
study through the 2019 Beginner Lecturer Research
grant.
REFERENCES
Azizah, D.N., Endang, K., dan Fahrauk, F. “Determination
of flavonoid levels of AlCl3 method in the cocoa peel
methanol extract (Theobroma cacao L.) . 2014Vol.
2(2): 45-49.
Azizah, D.N. dan Faramayuda, F., 2014. “Determination of
AlCl3 Method Flavonoid Levels in Methanol Extract of
The Skin of Cocoa Fruit (Theobroma Cacao L.).
Kartika Pharmaceutical Scientific Journal, 2 (2)
Chang,C.C., Ming-Hua Yang, Hwei-Mei Wen dan Jiing-
Chuan Chern. Estimation of total flavonoid content in
propolis by two complementary colorimetric methods.
Journal Of Food And Drug Analysis. 2002. Vol. 10. No.
3. 178-182
Claudea, Nusa., 2013., “ Antioxidants Extraction of
Bawang Dayak ( eleutherina palmifolia ) with
Ultrasonic Bath ( Study Type of Solvent and Extraction
Time ) “., Jurnal Pangan dan Agroindustri Vol. 5. No.
1, Malang : Jurusan Teknologi Hasil Pertanian, FTP
Brawijaya University.
Dimitrios, B., 2006., “ Source of Natural Phenolic
antioxidants Laboratory of Food Chemistry and
Technology”., School of Chemistry, Aristotle
University of Thessaloniki, 54124 Thessaloniki, J.
Food Sci 17 : 505 – 512
D Lestari et al, 2019, Antioxidant and anticancer activity of
Eleutherine bulbosa (Mill.) Urb on leukemia cells
L1210 J. Phys.: Conf. Ser. 1277 012022
Hambali, et al., 2005., “ Membuat Aneka Herbal Tea “,
Penebar Swadaya, Jakarta
Harborne, J.B. The phytochemical method, guiding the
modern way of analyzing plants, is the second issue.
Occurring from Phytochemical methods, by
Padmawinata, K., dan Soediro, I. Bandung : Penerbit
ITB Bandung. 1987. 13-15,69-102.
Herrera-Arellano, A., Flores-Romero, S., Chavez-Soto,
M.A. and Tortoriello, J. “Effectiveness and tolerability
of a standardized extract from Hibiscus sabdariffa in
patients with mild to moderate hypertension: a
controlled and randomizedclinical trial”.
Phytomedicine. 2004. 11(5), 375-382.
Humadi, S.S.,dan Viorica Istudor. Quantitative analysis of
bio- active compound in Hibiscus sabdariffa L. extracts.
Note I quantitative analysis of flavonoids. Farmacia.
2008. Vol. 56(6), 699-707.
Indrayani, S.” Validation of coloration of quercetin in
cream preparations by colorimetric with AlCl3 reagent
". Thesis. Yogyakarta: Sanata Dharma University.
2008. Hal:7,8,25.
Mun’in et al., 2008., “ Pembuatan Herbal Campuran
Kelopak Bunga Rosella dan Herba Seledri”, Depok :
University of Indonesia. Journals
Puspadewi, R., Adirestuti, P., & Menawati, R. Efficacy of
Dayak bulbs (Eleutherine palmifolia (L.) Merr.) As an
antimicrobial skin herb. Kartika: Pharmaceutical
Scientific Journal. 2013. 1(1), 31-37.
Pekal, A., & Pyrzynska, K. ”Evaluation of aluminium
complexation reaction for flavonoid content assay”.
Food Anal. Methods. 2014. Vol. 7, DOI
10.1007/s12161-014-9814-x, 1776-1782.
Puspadewi, R., Putranti Adirestuti, Rizka Menawati.
Efficacy of Dayak bulbs (Eleutherine palmifolia (L.)
Merr.) As an antimicrobial skin herb. Kartika
Pharmaceutical Scientific Journal. Faculty of
Pharmacy, Jenderal Achmad Yani University. 2013.
Vol.1.No.1, 31-37.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
362

Satheesh N.V.M, Kirti Singh. Smart ungual biopenetrant
from the roots of beta vulgaris. Journal of Applied
Pharmaceutical Research. 2017.5(2): 21–26.
Sepahpour, S., Selamat, J., Manap, M.Y., & Razis, A. F.
“Comparative analysis of chemical composition”.
Journal Molecules. 2018. Vol. 23 Ed 402, 2-17.
Yulia.R.& Putra.A “Teabag Formulation Packaging
Combination of Beet and Onion Bulbs "Research
Report. Bukittinggi: Mohammad Natsir University.
2019
Yuliani, S. dan Satuhu, S., 2012. A complete guide to
essential oils. Self-help spreader: Jakarta. Hal, 46.
Determination of Total Flavonoid Levels in Packaged Tea Bags Combination of Dayak Onion and Beet Root with UV Visible
Spectrophotometric Method
363
