
In Silico Dissection of the Drug Sensitivity of Mesothelioma Cell Lines
Archana Pal
1
and Vishal Singh Negi
1
School of Sciences, PP Savani University, Surat, Gujarat 394125, India
PP Savani University, NH 8, GETCO, Kosamba, Dhamdod, Surat, Gujarat 394125, India
Tel: +91 6355720256; Fax: +91-2612577300
Keywords: Pharmacogenomics GDSC, Mesothelioma, Drug sensitivity
Abstract: Malignant mesothelioma is an extremely aggressive cancer of the mesothelial cells. Asbestos exposure and
genetic predisposition are the two most well-established risk factors for mesothelioma occurrence. It has a
high mortality rate with poor prognosis and high chemotherapeutic resistance via unknown mechanisms. In
this study, we used in silico approach for studying the drug sensitivity response of 21 mesothelioma cell
lines from Genomics of Drug Sensitivity in Cancer’ (GDSC) database. We observed that only three cell
lines displayed sensitivity to various drugs. Among these three cell lines, two mesothelioma cell lines
displayed some commonalities in their drug sensitivities as well as their mutation profiles including,
mutation spectrums, the flanking regions of the mutated base, and their respective heatmaps.
1 INTRODUCTION
Malignant mesothelioma (MM) is an
aggressive cancer of the mesothelial cells with poor
prognosis (Zalcman et al. 2016) and ahigh mortality
rate (Carbone et al. 2012).The predicted number of
incidence is alarming; over 20 million people in the
US alone are on the verge of developing MM due to
asbestos exposure(Carbone et al. 2012) and the
global MM incidence and the resulting mortality
rates may be even higher for the developing nations
which happens to use significantly higher amount of
asbestos than the developed countries (Carbone et al.
2019).There have been several attempts to develop
drugs for MM using doxorubicin,
cyclophosphamide, cisplatin, carboplatin,
gemcitabine, pemetrexed, ethyl pyruvate, and
tremelimumab(Samson et al. 1987; Chahinian et al.
1993; Byrne et al. 1999; White et al. 2000; Kindler
et al. 2001; Hughes et al. 2002; Calabrò et al. 2013;
Pellegrini et al. 2017)in the past. However, the
majority of patients die within 24 months of
diagnosis often due to high chemotherapeutic
resistance via unknown mechanisms(Cortes-Dericks
et al. 2010; Mujoomdar et al. 2010; Tajima et al.
2010; Cregan et al. 2016). The effective treatment of
mesothelioma requires a multidimensional approach
such as finding novel targets and finding suitable
biomarkers for the resistant and sensitive cell lines.
In this study, we used in silico approach to dissect
the drug sensitivity of MM cell lines.
1.1 Primary Mesothelial Cell Lines
Primary cultures of mesothelial cells have
been established from rats, rabbits, mice, and
humans. Mesothelial cell lines provide several
advantages for experimental studies: they provide a
large number of cells isolated from a single donor,
cell lines can be isolated from genetically engineered
mice, and primary cell lines limit the number of
animals required for experiments. However, cell
lines have several disadvantages: variability among
donors, variability in culture conditions in different
laboratories, potential phenotypic and genetic
instability, and a limited life span in vitro. Some of
these disadvantages can be overcome by quality
control procedures.
For example, cell lines should not be
passaged indefinitely; frozen stocks should be
maintained and thawed at regular intervals to
prevent phenotypic and genetic instability. As in all
cell culture models, precautions are required to
prevent cross-contamination and contamination with
bacteria or viruses. DNA profiles could be useful to
identify cell lines; for example Manning et al
established initial
genetic profiles for their panel of human
malignant mesothelioma cell lines. All cultures
should be screened for Mycoplasma and other
pathogens (Masters, et al. 2000).
Technical details regarding primary human
mesothelial cell cultures have been summarized by
Pal, A. and Negi, V.
In Silico Dissection of the Drug Sensitivity of Mesothelioma Cell Lines.
DOI: 10.5220/0009512103250332
In Proceedings of the International Conference on Health Informatics and Medical Application Technology (ICHIMAT 2019), pages 325-332
ISBN: 978-989-758-460-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
325

Versnel et al (Versnel,et al. 1994) and Gerwin
(Gerwin, et al. 1994). Briefly, primary human
mesothelial cells require enriched culture media
supplemented with 10% to 20% fetal bovine serum,
exogenous growth factors [usually epidermal growth
factor (EGF)], insulin, transferrin, and
hydrocortisone. Rabbit, mouse, and rat primary
mesothelial cells require similar growth conditions,
with the important exception that growth of rat
pleural mesothelial cells is inhibited by EGF. As
reviewed by Walker et al , there are additional
differences in expression of growth factors and their
receptors between human and rat mesothelial cells.
Differences in growth factor responses have been
described in primary human mesothelial cell cultures
derived from different donors (Lechners, et al.
1989).
Mesothelial cell cultures have been
characterized by morphology, electronmicroscopy,
immunocytochemistry, and cytogenetic). Although
mesothelial cells can form monolayers with
epithelial morphology, this growth pattern can be
altered in vitro as described below.
At the ultrastructural level, mesothelial cells
typically show surface microvilli, abundant
mitochondria, extensive rough endoplasmic
reticulum, perinuclear intermediate filaments,
desmosomes, and tight junctions. Immuno
cytochemistry is useful to confirm expression of
markers specific for mesothelial cells, especially
coexpression of intermediate filaments, keratin, and
vimentin (Mackay, et.al. 1987) and expression of the
Wilms’ tumor suppressor gene, WT1 (Walker, et
al. 1994). These markers are also useful for the
immunohistochemical diagnosis of human malignant
mesotheliomas (Zeng, et al, Ordozen, et al. 2002).
Cytogenetic studies of human mesothelial cell lines
reveal a normal karyotype that may acquire
abnormalities after several passages (Versnel, et al.
1994). One primary murine mesothelial cell line has
been reported that spontaneously acquired a point
mutation in exon 5 of the p53 tumor suppressor
gene. This mutation increased growth rate in vitro;
however, it did not confer tumorigenicity (Cistulli, et
al. 1992).
Primary cell lines provide a valuable model
to study the cell biology and differentiation of
normal mesothelial cells. Primary cultures have also
been used to investigate the toxicologic effects of
asbestos and man-made mineral fibers (Lechner, et
al. 1991).
The mesothelium is derivedembryologically
from the mesoderm. At approximately embryonic
day 7.5 in the mouse, epithelial cells undergo
mesenchymal differentiation to form the mesoderm
cell layer. This morphologic differentiation is
governed by transcription factors snail and slug that
modulate expression of cadherins and cytoskeletal
proteins characteristic of mature mesothelial cells
(Carver, et al. 2001). In response to mechanical
injury, peritoneal dialysis, or chronic inflammation,
mesothelial cells also revert from an epithelial to a
mesenchymal phe- notype. This transdifferentiation
is termed the epithelial-mesenchymal transition and
has been investigated in primary cultures of human
mesothelial cells isolated from reactive peritoneal
effusions or dialysis effluent. In these pathologic
conditions, human mesothelial cells detach from the
mesothelial monolayer and survive in suspension.
When these reactive mesothelial cells are
placed in monolayer culture, they express epithelial
or mesenchymal phenotypes (Carver, at al. 2001)
characterized the expression of cytoskeletal proteins
including actin, vimentin, and several cytokeratins
by mesothelial cells isolated from ascitic fluid.
Modulation of the epithelial phenotype in vitro
depended on culture conditions: serum, EGF, and
hydrocortisone induced a mesenchymal phenotype,
while supplementation with retinoic acid induced an
epithelial phenotype. The epithelial– mesenchymal
transition of reactive human mesothelial cells in
vitro is characterized by reduced expression of some
cell surface proteoglycans (syndecan-4, glypican-1),
the WT1 tumor suppressor gene, and decreased
expression of E cadherin in parellel with expression
of the transcription factor snail. Transdifferentiation
of omental mesothelial cells in vitro was also
induced by mechanical wounding of mesothelial
monolayers or by exposure to the inflammatory
mediators, transforming growth factor-b1 (TGF-b1)
or interleukin-1b (IL-1b) (Carver, at al. 2001).
Mesothelial cells are sensitive target for
transformation by asbestos fibers. The biologic basis
for this increased sensitivity is unknown. Studies
conducted with cell culture models have provided
evidence that the iron-catalyzed generation of
reactive oxygen species is a plausible mechanism for
asbestos carcinogenicity. Reactive oxygen species
have been implicated in asbestos-induced apoptosis,
chromosomal damage, oxidative DNA damage, and
DNA strand breaks (Ollikainen, et al. 1996) in
human and rat pleural mesothelial cells. Variations
in antioxidant defense mechanisms have been
hypothesized to contribute to pulmonary disease
induced by fibers and particulates (Driscoll, et al.
2002). The antioxidant defense pathways of primary
rat pleural mesothelial cells have been characterized
in detail; these cultures have low catalase activity
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
326

and depend primarily on the glutathione pathway for
protection against oxidant stress (Kinnula, et al.
1992). These mechanistic studies suggest that
mesothelial cells are highly susceptible to DNA and
chromosomal damage in response to asbestos
exposure. Mesothelial cells with asbestos-induced
DNA damage that escape apoptosis may be
precursors for the development of malignant
mesothelioma (Broaddus, et al. 1996).
2 METHOD
2.1 Mesothelioma Cell Lines
The list of mesothelioma cell lines and their
respective COSMIC ids were obtained from the
Genomics of Drug Sensitivity in Cancer’ (GDSC)
database.
2.2 Datasets for Drug Response
The GDSC datasets are generated as a result
of various projects and are categorized into two
datasets, GDSC1 and GDSC2. The original dataset
of GDSC was expanded in the form of GDSC1 by
integrating heterogeneous molecular data of 11,289
tumors and 1,001 cell lines and measuring the
response of 1,001 cancer cell lines to 265 anti-cancer
drugs (Iorio et al. 2016) jointly by Wellcome Sanger
Institute and Massachusetts General Hospital
between 2009 and 2015. In contrast, the GDSC2
dataset was generated by Wellcome Sanger Institute
using the improved methods for screening and
assays. Considering that GDSC2 a more reliable
dataset, all our data are obtained from it and not
from GDSC1.
2.3 Chemical Structures of Drugs
The chemical structures of the sensitive drugs
were obtained from the Inxight: Drugs portal of the
National Center for Advancing Translational
Sciences (NCATS).
2.4 Mutation Spectrums
The mutations spectrum of the cell lines were
obtained from the ‘catalogue of somatic mutations in
cancer (COSMIC) portal. The mutation spectrum
plot displays all the substitution nucleotide base pair
changes on the Y-axis and the frequency on X-axis.
It shows the frequency of six substitution classes
(C:G>A:T, C:G>G:C, C:G>T:A, T:A>A:T,
T:A>C:G & T:A>G:C) and indels (which is used for
insertion or deletion of bases in the genome).
2.5 Flanking Regions of Mutated Bases
The flanking sequence for all mutations
referenced to the pyrimidine base (T>X, T>G, T>C,
T>A: C>X, C>T, C>G, C>A) for each cell lines
were obtained from the COSMIC portal available at
Sanger web server. It displays the mutated base at
position 0 together with the frequency for the 10
bases at the upstream and downstream of the
mutated base.
2.6 Genomic Heatmaps of
Mesothelioma Cell Lines
The genomic heatmaps from the cell line
projects were obtained for NCI-H2795, NCI-H513,
and MSTO-211H cell lines. These heatmaps were
constructed from counts of each mutation-type at
each mutation context corrected for the frequency of
each trinucleotide in the coding region of the
reference genome. The plot shows the log-
transformed values of these ratios. The 5′ base to
each mutated base is shown on the vertical axis and
3′ base on the horizontal axis.
3 RESULT
3.1 Mesothelioma Cell Lines in GDSC
The ‘Genomics of Drug Sensitivity in
Cancer’ (GDSC) database allows access to the drug
sensitivity datasets on a large number of 1001 cell
lines of which 990 cells lines have drug response
data available (Supplementary materials S1). Among
1001 cell lines, 21 belong to mesothelioma cancer
type. These cell lines include NCI-H2369, NCI-
H2373, NCI-H2461, NCI-H2591, NCI-H2595, NCI-
H2722, NCI-H2731, NCI-H2795, NCI-H2803, NCI-
H2804, NCI-H2810, NCI-H2818, NCI-H2869, NCI-
H290, NCI-H513, NCI-IST-MES1, NCI-MPP-89,
NCI-MSTO-211H, NCI-H2052, NCI-H2452, and
NCI-H28.
3.2 Drugs Sensitivity Response of
Mesothelioma Cell Lines and Their
Target Pathways
In the GDSC2 dataset majority of
mesothelioma cell lines (17 out of 21) including,
In Silico Dissection of the Drug Sensitivity of Mesothelioma Cell Lines
327
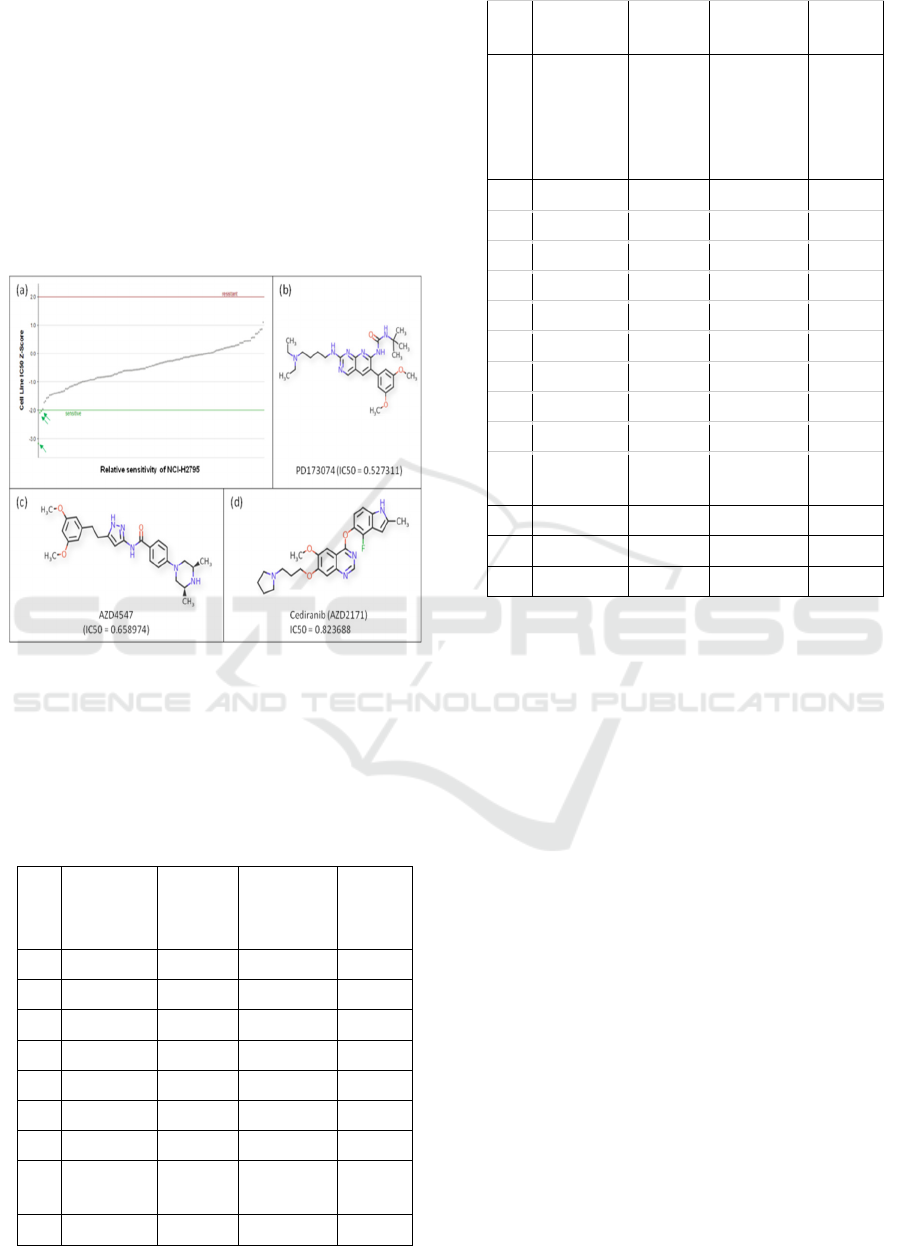
NCI-H2369, NCI-H2373, NCI-H2461, NCI-H2591,
NCI-H2595, NCI-H2722, NCI-H2731, NCI-H2803,
NCI-H2804, NCI-H2810, NCI-H2818, NCI-H2869,
NCI-H290,NCI-IST-MES1, NCI-MPP-89, NCI-
H2052, NCI-H2452, and NCI-H28 exhibited no
sensitivity to any drugs. In contrastonly 3 of the
mesothelioma cell lines including NCI-H2795,NCI-
H513, and MSTO-211Hexhibited sensitivity to
different drugs. The cell line NCI-H2795 was
sensitive to three different drugs PD173074,
AZD4547, and Cediranib (Table 1, Fig. 1).
Figure 1: Drug sensitivity of mesothelioma cell lines NCI-
H2795 (a). The cell line is sensitive to PD173074 (b),
AZD4547, and (c) Cediranib.
Whereas, the other two cell lines NCI-H513, and
MSTO-211H are sensitive to Acetalax, and
PD173074, respectively (Table 1, Fig. 2).
Table 1: Drug sensitivity of mesothelioma cell lines
S.No. Cell Lines
Sensitivity
to Drugs
Targets IC50
1 NCI-H2369 - - -
2 NCI-H2373 - - -
3 NCI-H2461 - - -
4 NCI-H2591 - - -
5 NCI-H2595 - - -
6 NCI-H2722 - - -
7 NCI-H2731 - - -
8 NCI-H2795 PD173074
FGFR1,
FGFR3 0.527311
AZD4547 FGFR1, 0.658974
FGFR2,
FGFR3
Cediranib
VEGFR,
FLT1, FLT2,
FLT3, FLT4,
KIT,
PDGFRB 0.823688
9 NCI-H2803 - - -
10 NCI-H2804 - - -
11 NCI-H2810 - - -
12 NCI-H2818 - - -
13 NCI-H2869 - - -
14 NCI-H290 - - -
15 NCI-H513 Acetalax - 1.084383
16 IST-MES1 - - -
17 MPP-89 - - -
18
MSTO-
211H PD173074
FGFR1,
FGFR3 2.17617
19 NCI-H2052 - - -
20 NCI-H2452 - - -
21 NCI-H28 - - -
Thecell line NCI-H2795 was found to be
sensitive for three different drugsPD173074,
AZD4547, and Cediranib. The PD173074 is
inhibitory to FGFR1, and FGFR3; AZD4547 inhibits
FGFR1, FGFR2, and FGFR3. The interesting
commonality between the two drugs is that both
inhibitfibroblast growth factor receptors (FGFRs)
thereby inhibiting thesignal transduction pathways,
and, so, the inhibition of tumor cell proliferation and
tumor cell death. Up-regulation of FGFR, which is a
tyrosine kinase receptor, has been reported in many
tumors, and the sensitivity of NCI-H2795 to the
drugs PD173074 and AZD4547 suggests theover-
expression of FGFRs as the major driving force for
mesothelioma. Similarly, drug cediranib is a potent
inhibitor of vascular endothelial growth factor
(VEGF) receptor tyrosine kinases. Considering the
targets of all these three drugs for which NCI-H2795
is sensitive, it is conceivable that the tyrosine kinase
receptors such as FGFRs and VEGF are over-
expressed in mesothelioma and are essential to
tumor cellular proliferation, differentiation and
survival.Like NCI-H2795, the cell line MSTO-
211His also sensitive to the PD173074, the FGFRs
inhibitor. In contrast, the NCI-H513cell line is
sensitive to Acetalax, which is a laxative and its
specific target is largely unknown. However,
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
328
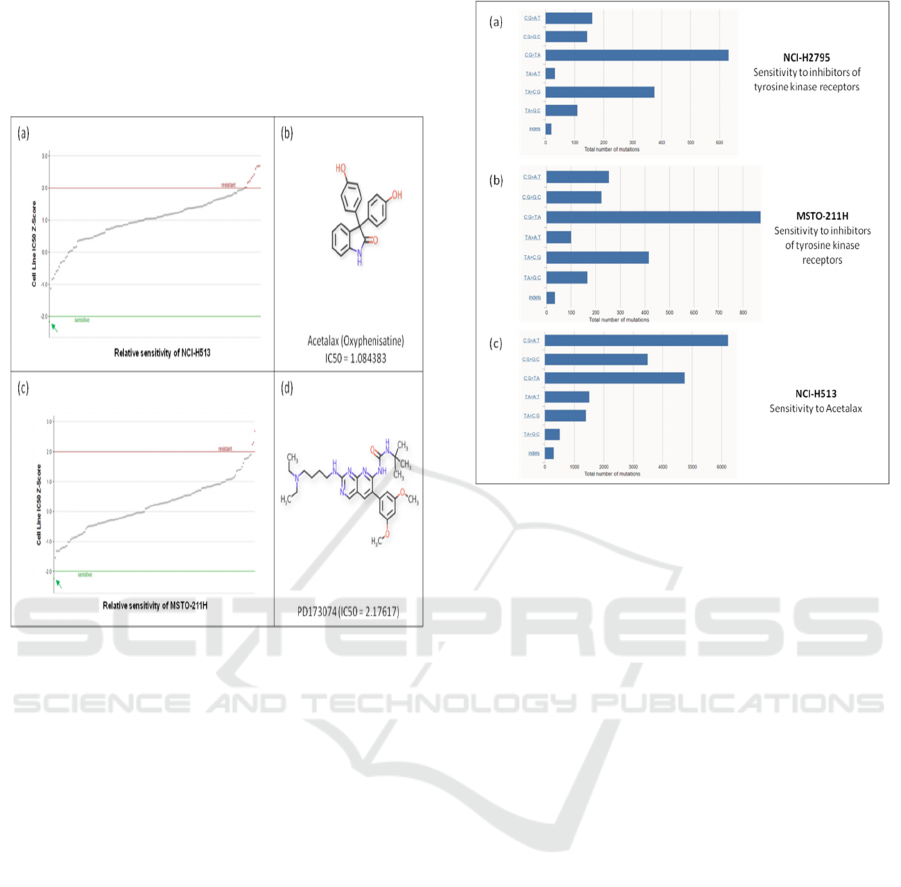
Acetalax has been shown to trigger a cell starvation
response leading to autophagy, mitochondrial
dysfunction, and autocrine TNFα-mediated
apoptosis(Morrison et al. 2013).
Figure 2: Drug sensitivity of mesothelioma cell lines NCI-
H513 and MSTO-211H. (a-b) NCI-H513 is sensitive to
Acetalax. (c-d) MSTO-211H is sensitive to PD173074.
3.2 Mutation Spectrum of Sensitive
Cell Lines
The mutation spectrum of cell lines NCI-
H2795 and MSTO-211H displays some degree of
similarity. In both cases, the frequency of C:G>T:A
substitution is 630 and 871, respectively, which are
highest among all the different substitution classes.
Additionally, the class of the second most frequent
substitution in both the cell line is also the same; the
T:A>C:G substitution in NCI-H2795 and MSTO-
211H is 375 and 415, respectively (Fig. 3a and b). In
contrast to NCI-H2795 and MSTO-211H, the class
of the most frequent substitution in NCI-H513 is
C:G>A:T followed by C:G>T:A, though the number
of total mutations in each substitution class in NCI-
H513 is significantly higher compared to the other
two cell lines (Fig. 3c).
Figure 3: Mutation spectrum of mesothelioma cell
lines NCI-H2795, MSTO-211H, and NCI- H513.
3.3 Flanking Regions of Mutated Base
Apart from the mutation spectrum, the
flanking sequence for all mutations referenced to the
pyrimidine base (T>X, T>G, T>C, T>A: C>X, C>T,
C>G, C>A) for each cell lines were also analyzed to
test if there is any similarity in the cell lines NCI-
H2795 and MSTO-211H. Interestingly, we observed
that thenucleotide frequency of ten bases upstream
and downstream of the T>X and C>X mutations
were maximum for T>C and C>T, respectively for
both NCI-H2795 and MSTO-211H (Fig. 4). As
conceivable, the nucleotide frequency of ten bases
upstream and downstream of the T>X and C>X
mutations were maximum for T>A and C>A for the
cell line NCI-H513 (Fig. 5).
In Silico Dissection of the Drug Sensitivity of Mesothelioma Cell Lines
329
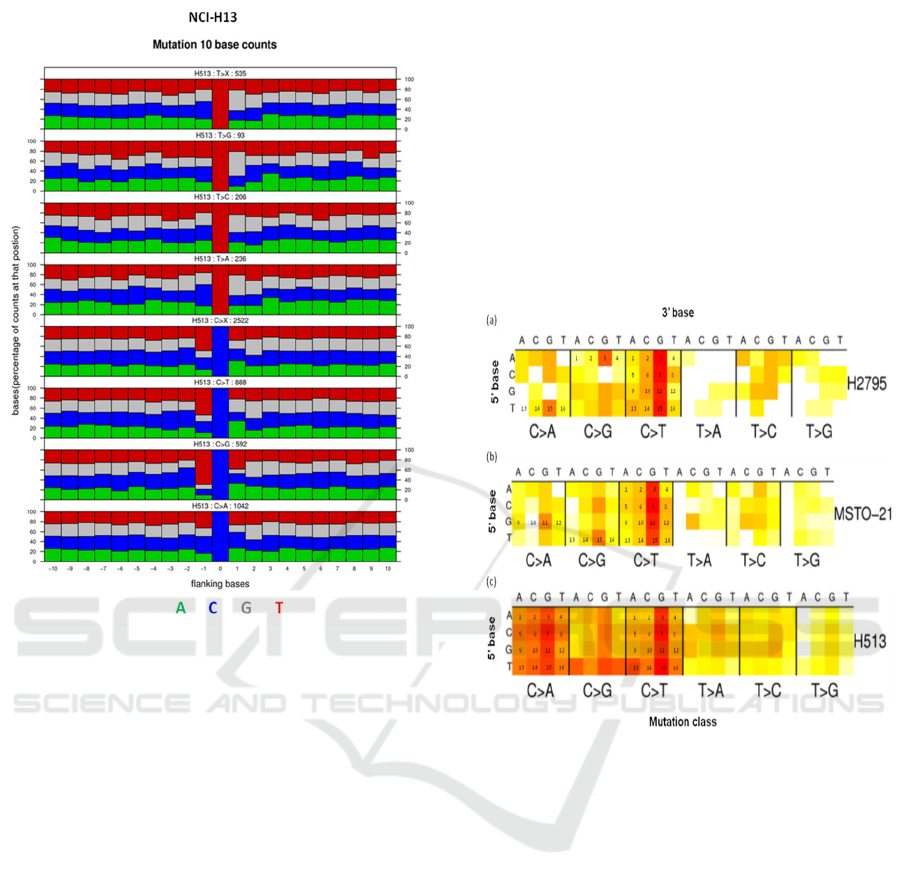
Figure 5: Extended sequence context diagram of
mesothelioma cell line NCI-H513.
The plots shows 21bp sequence context,
combining data from all mutations in a single
sample. The nucleotide frequencies of ten bases
upstream and downstream of the mutated base are
shown normalised to the frequency across the coding
region of the genome.
3.4 Genomic Heatmaps of
Mesothelioma Cell Lines
The deamination of cytosine in a CpG
dinucleotide context (emphasized by 3, 7, 11, and 15
of the mutation class C>T) is one of the common
features of the genomic heatmaps of all the three
mesothelioma cell lines, NCI-H2795, MSTO-211H,
and NCI-H513 (Fig. 6).
The genomic heatmaps of NCI-H2795 and MSTO-
211H were observed to be quite similar to each other
(Fig. 6a and b). The XpCpA and XpCpT
(emphasized by 1, 5, 4 and 12 in Fig. 6a and by 3, 9,
and 14 in Fig. 6b) are rarely mutated in NCI-H2795
and MSTO-211H (Fig. 6a and b). In the mutation
class C>A, the TpCpG (emphasized by 15 of the
mutation class C>A in Fig. 6a) of NCI-H2795 and
the GpCpG (emphasized by 11 of the mutation class
C>A in Fig. 6b) of MSTO-211H are frequently
mutated. Additionally, in the mutation class C>G,
the triplet ApCpG (emphasized by 3 of the mutation
class C>G in Fig. 6a) of NCI-H2795 and the TpCpG
(emphasized by 15 of the mutation class C>G) in
MSTO-211H have frequently mutated bases.
Moreover, the mutation classes T>A and T>G are
rarely mutated in both NCI-H2795 and MSTO-
211H.In contrast to NCI-H2795 and MSTO-211H,
the cell line NCI-H513 exhibited a high frequency of
mutations in C>A and C>G classes (Fig. 6c).
Figure 6: Genomic heatmap of mesothelioma cell
lines NCI-H2795, MSTO-211H, and NCI-H513.
The heatmap shows the frequency of
mutations for all possible triplet bases normalised
against the frequency across the coding genome.
These triplets are composed of the mutated base
together with the 5’ and 3’ bases. There are 96
possible triplets, 16 for each mutation class (C>A,
C>G, C>T, T>A, T>C, and T>G).
5 CONCLUSION
A vast majority of mesothelioma cell lines in
the GDSC database did not display sensitivity to any
of the drugs tested so far. Drug response data shows
that only three cell lines including NCI-H2795, NCI-
H513, and MSTO-211H exhibitedsensitivity to
different drugs. The NCI-H2795 was sensitive to
PD173074, AZD4547, and cediranib, while MSTO-
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
330

211H and NCI-H513 cell lines are sensitive to
PD173074, and acetalax, respectively.
Interestingly, the targets of the
drugsPD173074, AZD4547, and cediranib are
tyrosine kinase receptors such as FGFRs and VEGF
suggesting that the tyrosine kinase receptors in the
two mesothelioma cell lines, NCI-H2795 and
MSTO-211H, are essential to tumor cellular
proliferation, differentiation, and survival. Unlike
NCI-H2795 and MSTO-211H, the NCI-H513 cell
line is sensitive to Acetalax (and resistant to other
drugs), which is a laxative and its specific target is
largely unknown.
It is conceivable that the genomic mutation
profile of the cell lines, NCI-H2795 and MSTO-
211H, which display similarity in their response to
drugs, is likely similar. Therefore, we also looked
into the mutation spectrum, flanking regions of the
mutated bases, and the heatmaps of the substitution
mutations of these cell lines. As expected these
displayed a very similar mutation profile, which is
strikingly different that of the NCI-H513. This
information along with any future study involving
the study of the transcriptomic profile of resistant
and sensitive cell lines could provide us with
suitable biomarkers for drug sensitivity response.
ACKNOWLEDGEMENTS
Vishal Negi and Archana Pal Negi are
thankful to Dr. ParagSanghani (Provost, PP Savani
University) and ShriVallabhbhaiSavani (President,
PP Savani University) for their support and
providing bioinformatics facility to carry out this
study. Vishal Negi is also thankful to the Dr. Saiful
Anwar Matondang and the organizers of the
International Conference on Health Informatics,
Medical and Application Technology (ICHIMAT-
2019) for inviting him as a keynote speaker.
REFERENCES
Broaddus VC, Yang L, Scavo LM, et al. Asbestos induces
apoptosis of human and rabbit pleural mesothelial
cells via reactive oxygen species. J Clin Invest
1996;98:2050–2059.
Byrne, M. J., J. A. Davidson, A. W. Musk, J. Dewar, G.
van Hazel, M. Buck, N. H. de Klerk, and B. W.s.
Robinson. 1999. Cisplatin and Gemcitabine Treatment
for Malignant Mesothelioma: A Phase II Study.
Journal of Clinical Oncology 17: 25–25.
https://doi.org/10.1200/JCO.1999.17.1.25.
Calabrò, Luana, Aldo Morra, Ester Fonsatti,
OrnellaCutaia, Giovanni Amato, Diana Giannarelli,
Anna Maria Di Giacomo, et al. 2013. Tremelimumab
for patients with chemotherapy-resistant advanced
malignant mesothelioma: an open-label, single-arm,
phase 2 trial. The Lancet Oncology 14: 1104–1111.
https://doi.org/10.1016/S1470-2045(13)70381-4.
Carbone, Michele, Prasad S. Adusumilli, H. Richard
Alexander, Paul Baas, Fabrizio Bardelli, Angela
Bononi, Raphael Bueno, et al. 2019. Mesothelioma:
Scientific clues for prevention, diagnosis, and therapy.
CA: A Cancer Journal for Clinicians 69: 402–429.
https://doi.org/10.3322/caac.21572.
Carbone, Michele, Bevan H. Ly, Ronald F. Dodson, Ian
Pagano, Paul T. Morris, Umran A. Dogan, Adi F.
Gazdar, Harvey I. Pass, and Haining Yang. 2012.
Malignant mesothelioma: Facts, Myths, and
Hypotheses. Journal of Cellular Physiology 227: 44–
58. https://doi.org/10.1002/jcp.22724.
Carver EA, Jiang R, Lan Y, et al. The mouse snail gene
encodes a key regulator of the epithelial-mesenchymal
transition. Mol Cell Biol 2001;21: 8184–8188.
Chahinian, A P, K Antman, M Goutsou, J M Corson, Y
Suzuki, C Modeas, J E Herndon, J Aisner, R R
Ellison, and L Leone. 1993. Randomized phase II trial
of cisplatin with mitomycin or doxorubicin for
malignant mesothelioma by the Cancer and Leukemia
Group B. Journal of Clinical Oncology 11:1559–1565.
Cistulli CA, Sorger T, Marsella JM, et al. Spontaneous
p53 mutation in murine mesothelial cells: increased
sensitivity to DNA damage induced by asbestos and
ionizing radiation. Toxicol Appl Pharmacol
1996;141:264–271.
Cortes-Dericks, Lourdes, Giovanni L. Carboni, Ralph A.
Schmid, and Golnaz Karoubi. 2010. Putative cancer
stem cells in malignant pleural mesothelioma show
resistance to cisplatin and pemetrexed. International
Journal of Oncology 37: 437–444.
https://doi.org/10.3892/ijo_00000692.
Cregan, Sian, Lauran Mc Donagh, Yun Gao, Martin P.
Barr, Kenneth J. O’Byrne, Stephen P. Finn, Sinead
Cuffe, and Steven G. Gray. 2016. KAT5 (Tip60) is a
potential therapeutic target in malignant pleural
mesothelioma. International Journal of Oncology
48:1290–1296. https://doi.org/10.3892/ijo.2016.3335.
Driscoll KE, Carter JM, Borm PJA. Antioxidant defense
mechanisms and the toxicity of fibrous and nonfibrous
particles. Inhal Toxicol 2002;14: 101–118.
Gerwin BI. Mesothelial carcinogenesis: possible avenues
of growth promotion. In: Jaurand M-C, Bignon J, eds.
The Mesothelial Cell and Mesothelioma. New York:
Marcel Dekker, 1994:223–244.
Hughes, Andy, Paula Calvert, Ashraf Azzabi, Ruth
Plummer, Rob Johnson, Jim Rusthoven, Melanie
Griffin, et al. 2002. Phase I Clinical and
Pharmacokinetic Study of Pemetrexed and Carboplatin
in Patients With Malignant Pleural Mesothelioma.
Journal of Clinical Oncology 20: 3533–3544.
https://doi.org/10.1200/JCO.2002.10.073.
In Silico Dissection of the Drug Sensitivity of Mesothelioma Cell Lines
331

Iorio, Francesco, Theo A Knijnenburg, Daniel J Vis,
Graham R Bignell, Michael P Menden, Michael
Schubert, NanneAben, et al. 2016. A landscape of
pharmacogenomic interactions in cancer.Cell 166:
740–754.
Kinnula VL, Everitt JI, Mangum JB, et al. Antioxidant
defense mechanisms in cultured pleural mesothelial
cells. Am J Respir Cell Mol Biol 1992;7: 95–103.
Kindler, Hedy L., Frederick Millard, James E. Herndon II,
Nicholas J. Vogelzang, Yasunosuke Suzuki, and Mark
R. Green. 2001. Gemcitabine for malignant
mesothelioma: A phase II trial by the Cancer and
Leukemia Group B. Lung Cancer 31: 311–317.
https://doi.org/10.1016/S0169-5002(00)00166-5.
Lechner JF, Gerwin BI, Reddel RR, et al. Studies on
human mesothelial cells: effects of growth factors and
asbesti-form fibers. In: Harris CC, Lechner JF,
Brinkley BR, eds. Cellular and Molecular Aspects of
Fiber Carcinogenesis. New York: Cold Spring Harbor
Laboratory Press, 1991: 115–130.
Mackay AM, Tracy RP, Craighead JE. Intermediate
filament proteins in asbestos-induced mesotheliomas
of the rat. Cancer Res 1987;47:5461–5468.
Manning LS, Whitaker D, Murch AR, et al. Establishment
and characterization of five human malignant
mesothelioma cell lines derived from pleural
effusions. Int J Cancer 1991;47:285–290.
Masters JRW. Human cancer cell lines: fact and fantasy.
Nature Rev Mol Cell Biol 2000;1:233–236.
Morrison, Bethanie L, Michael E Mullendore, Luke H
Stockwin, Suzanne Borgel, Melinda G Hollingshead,
and Dianne L Newton. 2013. Oxyphenisatin acetate
(NSC 59687) triggers a cell starvation response
leading to autophagy, mitochondrial dysfunction, and
autocrine TNFα-mediated apoptosis. Cancer Medicine
2: 687–700. https://doi.org/10.1002/cam4.107.
Mujoomdar, Aneil A., Tamara R. Tilleman, William G.
Richards, Raphael Bueno, and David J. Sugarbaker.
2010. Prevalence of in vitro chemotherapeutic drug
resistance in primary malignant pleural mesothelioma:
Result in a cohort of 203 resection specimens. The
Journal of Thoracic and Cardiovascular Surgery 140:
352–355. https://doi.org/10.1016/j.jtcvs.2009.11.072.
Pellegrini, Laura, JiamingXue, David Larson, Sandra
Pastorino, SandroJube, Kelly H. Forest, Zeyana Salim
Saad-Jube, et al. 2017. HMGB1 targeting by ethyl
pyruvate suppresses malignant phenotype of human
mesothelioma. Oncotarget 8: 22649–22661.
https://doi.org/10.18632/oncotarget.15152.
Samson, M K, L P Wasser, E C Borden, H J Wanebo, R H
Creech, M Phillips, and L H Baker. 1987. Randomized
comparison of cyclophosphamide, imidazole
carboxamide, and adriamycin versus
cyclophosphamide and adriamycin in patients with
advanced stage malignant mesothelioma: a Sarcoma
Intergroup Study. Journal of Clinical Oncology 5: 86–
91. https://doi.org/10.1200/JCO.1987.5.1.86.
Tajima, K., R. Ohashi, Y. Sekido, T. Hida, T. Nara, M.
Hashimoto, S. Iwakami, et al. 2010.Osteopontin-
mediated enhanced hyaluronan binding induces
multidrug resistance in mesothelioma cells. Oncogene
29: 1941–1951. https://doi.org/10.1038/onc.2009.478.
Ordonez NG. Immunohistochemical diagnosis of
epithelioid mesotheliomas: a critical review of old
markers, new markers. Hum Pathol 2002;33: 953–967.
Versnel MA, van der Kwast TH, Hoogsteden HC, et al.
Establishment and characteristics of human normal
and malignant mesothelial cell lines. In: Jaurand M-C,
Bignon J, eds. The Mesothelial Cell and
Mesothelioma. New York: Marcel Dekker, 1994:169–
186.
Walker C, Rutten F, Yuan X, et al. Wilms’ tumor
suppressor gene expression in rat and human
mesothelioma. Cancer Res 1994;54:3101–3106.
White, S. C., H. Anderson, G. C. Jayson, L. Ashcroft, M.
Ranson, and N. Thatcher. 2000. Randomised phase II
study of cisplatin-etoposide versus infusional
carboplatin in advanced non-small-cell lung cancer
and mesothelioma. Annals of Oncology 11: 201–206.
https://doi.org/10.1023/A:1008328605413.
Zalcman, Gérard, JulienMazieres, Jacques Margery,
Laurent Greillier, Clarisse Audigier-Valette, Denis
Moro-Sibilot, Olivier Molinier, et al. 2016.
Bevacizumab for newly diagnosed pleural
mesothelioma in the Mesothelioma Avastin Cisplatin
Pemetrexed Study (MAPS): a randomised, controlled,
open-label, phase 3 trial. The Lancet 387: 1405–1414.
https://doi.org/10.1016/S0140-6736(15)01238-6.
Zeng L, Fleury-Feith J, Monnet I, et al.
Immunocytochemical characterization of cell lines
from human malignant mesothelioma: characterization
of human mesothelioma cell lines by
immunocytochemistry with a panel of monoclonal
antibodies. Hum Pathol 1994;25:227–234.
ICHIMAT 2019 - International Conference on Health Informatics and Medical Application Technology
332
