
The Effect of Beeswax and Chitosan Concentrations as
Superhydrophobic Coating on Wound Dressing
Inggit Kresna Maharsih, Fadhil Muhammad Tarmidzi, Riza Alviany, Mela Aurelia,
Sisca Ardelia Putri
Institut Teknologi Kalimantan
Keywords: Beeswax, Chitosan, Superhydrophobic, Wound Dressing
Abstract: One of the factors that caused the wound dressing to be wet is water permeability through wound dressing
pores. A wet wound dressing must be replaced frequently in order to prevent infection. On the other side,
the recurring replacement of wound dressing increases the amount of infectious waste. As a consequence,
one of wound dressing called Hypafix is coated with the beeswax-chitosan mixture to obtain a waterproof
ability by a facile method. The purpose of this study is to determine the effect of concentrations of beeswax
and chitosan solutions as a superhydrophobic coating material on Hypafix and analyze the waterproof
characteristics. There are two models of research, beeswax concentration (0, 0.25, 0.5, 2, 2.5%wt/v) with
constant concentration of chitosan 0.5%wt/v, and chitosan concentration (0, 0.25, 0.5, 2, 2.5%wt/v) with
fixed concentration of beeswax 0.5%wt/v. The contact angle (θ), hysteresis, morphology of film, and
functional group analysis were characterized. The results showed that the contact angle was significantly
increased with increasing beeswax and chitosan concentrations but decreased at a concentration of 3%wt/v.
The lowest hysteresis of the sample was successfully obtained at 1.3° with θ~151.2° using beeswax/chitosan
concentration of (2.5 : 0.5) %wt/v. Scanning Electron Microscope (SEM) showed that the film covered the
gauze fibers. Hence the surface was rougher and also increased contact angle as explained in Cassie-Baxter
Theory. Furthermore, FTIR indicated that the layers formed on the fibre by both beeswax and chitosan
compounds, while they contributed to the Hypafix surface superhydrophobicity in beeswax and chitosan
optimum concentrations.
1 INTRODUCTION
Several strategies are needed for wound care. The
ability of the dressing to maintain the moisture of the
wound, absorb exudate, and remove dead tissue
must be considered (Setiyawan, 2016). The wound
that loses its moisture will cause damage to the
tissue, while for a wound that contains a lot of
exudates, an absorbent must be applied to keep the
moisture. The more exudates produced, then the
frequency for changing the bandages will be higher.
In addition, the moisture of dressing is affected by
the amount of water that hits the dressing. Water
seeping into the dressing will increase the moisture
inside and resulting in an increase in the frequency
of dressing replacements, even though the exudate
absorbed was still very low. Thus, a waterproof
patch is needed to reduce the intensity of the
dressing replacement.
In recent years, researchers have been
established modern wound dressing not only to
cover the wound but aid the function of the wound.
This dressing is focused both on keeping the wound
moisture and promote healing. One of modern
wound dressing can be classified as interactive
wound dressings. They are semi-occlusive or
occlusive that focused more on preventing the skin
from losing moisture by forming a protective film
over the epidermis (Degreef, 1998). Occlusive
dressings are widely used to support the wound
healing process. Occlusion develops the
microenvironment of a wound and increases the rate
of repithelialization (Fernandez-Castro et al.,
2017).
Hypafix
a
One of the most common modern
dressing is a white, thin, elastic, adhesive coated
a
Hypafix (Registered Trade Mark): supplied by BSN
Medical, Inc., Charlotte, NC, United States.
58
Maharsih, I., Tarmidzi, F., Alviany, R., Aurelia, M. and Putri, S.
The Effect of Beeswax and Chitosan Concentrations as Superhydrophobic Coating on Wound Dressing.
DOI: 10.5220/0009405300580063
In Proceedings of the 1st International Conference on Industrial Technology (ICONIT 2019), pages 58-63
ISBN: 978-989-758-434-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

non-woven polyester film permeable to both air and
moisture in order to minimize the risk of maceration.
This dressing still has hydrophilic properties, which
can cause water to be absorbed from the outside.
Water that contacts with a hydrophilic surface will
wet the surface when dripped on it. Wet wound
dressing can induce wound infection so that it
should be replaced often. However, the wound
dressing is one of infectious waste; hence its amount
has to be reduced as medical waste. In contrast,
when a surface has hydrophobic property, water will
not wet the surface (Wenten et al., 2015). Therefore,
a wettability property modification on a wound
dressing surface is conducted to reduce the amount
of infectious waste. A method is proposed to make a
waterproof dressing by modified its outer side to
become hydrophobic. One of the parameters that can
be measured to determine the hydrophobicity of a
material is the contact angle. Material, which has a
contact angle above 90
o
, can be categorized as
hydrophobic materials (Butruk et al., 2011). If the
contact angle exceeds 150
o
, the material can be
categorized into superhydrophobic material. By
forming a superhydrophobic layer, it will be difficult
for water to seep through the pore pads.
Commercially, there are some waterproof wound
dressings that are widely used in the world. Mostly,
those kinds of dressings have hydrophobic abilities
due to Durable Water Repellent (DWR) coating on
their fabric. However, in general, a key substance
resulting in a waterproof surface is contained fluor.
Based on previous studies, the fluor-based
substances are hazardous for human health, so their
application on wound dressing is not suggested. On
the other hand, Chemical Vapor Deposition (CVD)
method provides a very thin water repellant film
with no use of hazardous regeants. Nonetheless, this
method is categorized as a sophisticated method and
needs a vacuum condition. Based on the
background, a facile method using no harmful
material is induced to modify the surface of the
wound dressing. A mixture of beeswax and chitosan
can be used as one of the candidates to form a
hydrophobic coating material. El-Bisi reported that
the addition of beeswax into chitosan on cotton
fabric could increase the contact angle of up to 152
o
(El-Bisi et al., 2016). Beeswax and chitosan have
anti-microbial properties that are widely used for
medical purposes.
Beeswax is a pure wax formed from a beehive
derived from Apis mellifera bees, which contain
ester almost 70%. Esters have a non-polar group that
has the ability to bind water relatively small. A study
has been reported that beeswax has a contact angle
between 100
o
-110
o
(Naderizadeh et al., 2019).
Chitosan has hydroxyl and amine groups along
the chain. This causes chitosan to be very effective
for adsorbing cations from organic substances,
especially proteins and fats (Lee et al., 2001).
Moreover, chitosan polymer contains a positively
charged amino group that can bind to negatively
charged via ionic or hydrogen bond. These bonds
cause chitosan to be difficult to bind to water
(Setiani et al., 2013). It has been reported that
chitosan has a contact angle between 70
o
-91
o
(Farris
et al., 2011).
In the present work, we describe the effect of
chitosan and beeswax mixture concentration to
develop a superhydrophobic coating on Hypafix.
The morphology, hysteresis, and contact angle were
studied in detail.
2 EXPERIMENTAL METHODS
2.1 Synthesis of Superhydrophobic
Layer
Beeswax (Apis mellifera) solution was prepared by
dissolving beeswax in 2-propanol (Merck, 98%
purity). Prior to use, 2-propanol was preheated for
30 minutes at 75°C. Moreover, chitosan (degree of
deacetylation = 79%) solution was prepared to
dissolve in 1% (v/v) acetic acid solution (Merck,
98% purity) at room temperature with a stirring
speed of 1000 rpm for 1 hour and further heated for
30 minutes at 75°C. Then, beeswax solution and
chitosan solution are mixed at 75°C with a stirring
speed of 1200 rpm for 2 hours. After that, the whole
mixture was homogenized for 30 minutes by
Ultrasonic. Last, Carboxymethyl Cellulose (CMC)
with high molecular weight (262.19 g mol
-1
) added
in the mixture, and homogenization is continued for
60 min. The mixture was obtained from two
experiment models. First, different concentration of
beeswax solution (0.25%, 0.5%, 2%, 2,5%) in wt/v
were mixed with fixed concentration of chitosan
solution (0.5% wt/v). Second, different
concentration of chitosan solution (0.25%, 0.5%,
2%, 2,5%) in wt/v were mixed with fixed
concentration of beeswax solution (0.5% wt/v).
2.2 Coatings Preparation
The mixture coatings were prepared by the casting
method in the outer layer of gauze (Hypafix). After
the deposition process, the samples were dried at
The Effect of Beeswax and Chitosan Concentrations as Superhydrophobic Coating on Wound Dressing
59

room temperature for 20 hours. Thus, the patch with
coatings was obtained.
2.3 Coatings Characterization
2.3.1 Contact Angle Measurements
To evaluate the surface hydrophobicity of patch with
coatings, contact angles were determined by
dropping ten microliters of water on the surface of
the patch that has been coated with
superhydrophobic material. Then, the picture of the
surface of the patch was taken using a camera
(Nikon D5500), and the results of the images were
analyzed using ImageJ software to determine the
right and left contact angle values. The reported
contact angle results were the average values from
the right and left contact angle values. Measurement
was performed three times.
2.3.2 Contact Angle Hysteresis
Measurements
The contact angle hysteresis measurements have the
same procedure as the one previously described for
contact angle measurements. However, the
hysteresis measurements were not carried out on a
flat surface, but rather a surface with an angle of 10°
from the flat surface. Thus, the values of the front
angle and rear angle are obtained. The reported
contact angle results in the difference between the
two angles.
2.3.3 Morphology
In order to know the surface of the patch which is
coated with a mixture of beeswax-chitosan, the
patch with coatings were visualized using a scanning
electron microscope (SEM Tescan Vega3 LMU) at
PT Gestrindo Sakti Utama, Jakarta
2.3.4 Functional Group
Functional group analysis was performed to
determine the functional group on the beeswax-
chitosan mixture. The analysis was carried out using
Fourier Transform Infrared Spectroscopy (FTIR
PerkinElmer type Spectrum Two) at PT DKSH
Indonesia, Jakarta.
3 RESULTS AND DISCUSSION
3.1 Characteristic of the Coating
Solution
Naturally, beeswax consists of no polar groups in its
structure. This characteristic makes beeswax is
difficult to bind water molecules. On the other hand,
chitosan reduces the water affinity that results in no
permeation of drops on the substrate (Mohamed et
al., 2011). When beeswax and chitosan are mixed,
the hydrophobic behavior is improved to be
superhydrophobic. Superhydrophobic ability is
produced by bonding between NH
3
+
groups from
chitosan and fatty acids from beeswax that has
anions. This bond is categorized as an ionic bond. In
order to form NH
3
+
polycationic, chitosan has been
dissolved in acetic acid 1% v/v. Carboxymethyl
cellulose (CMC), as an emulsifier, is needed to
homogenize the beeswax-chitosan mixture. After
sonication, beeswax droplets in the solution become
smaller, and they are adsorbed by CMC. This
mechanism prevents the coalescence of small
droplets from being the bigger ones (Dickinson,
2009).
3.2 Wetting Properties
The ordinary Hypafix is permeable to water, as
shown in Fig. 1. Drops come through pores, and it
corresponds to the increase of moisture content in
wound dressing. Besides, a wet wound dressing
should be replaced with the new one. Frequent
replacement of wound dressing can lead to
infectious waste pollution
Figure 1. The water drop behavior on the ordinary Hypafix
ICONIT 2019 - International Conference on Industrial Technology
60
In contrast, when Hypafix is coated by beeswax-
chitosan mixture, drops permeation does not exist,
and the drops have a static contact angle on a flat
plane. The variations of beeswax and chitosan
concentrations evidently affect the coating
wettability properties. They are summarized in Fig.2
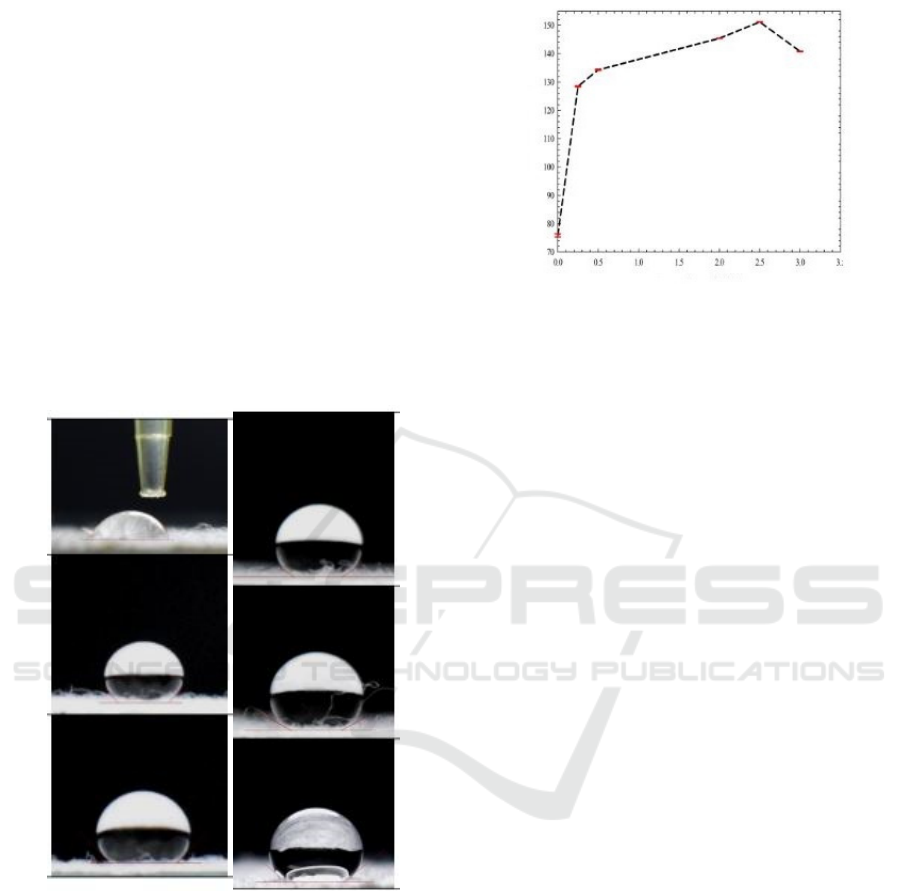
and Fig.3. In Fig. 2, beeswax concentration varies
from 0 to 3% wt/v. The increase of beeswax
concentration leads to a higher contact angle due to
many fatty acids compounds provided by the
beeswax bond with NH
3
+
groups. The highest
contact angle,151o, is resulted from 2,5% wt/v of
beeswax. However, the contact angle decreases to
140
o
when beeswax is added up to 3% wt/v. This
reduction relates to NH
3
+
groups' lack of availability
to bond with fatty acids, so the superhydrophobicity
is majorly contributed from the nonpolar beeswax
structure (Supeni and Irawan, 2012).
(a)
(b)
Figure.2. (a) The variations of beeswax concentrations and
the fixed chitosan concentration (0.5%w/v) affect the
contact angles. (b) Graphic of contact angles and beeswax
concentrations.
The other result is conducted from variations of
chitosan concentration and fixed beeswax
concentration (0,5% wt/v), as shown in Fig 3. The
high concentration of chitosan refers to many NH
3
+
groups. These groups bond with anions of fatty acids
to modify the wettability from hydrophobic to
superhydrophibic. The contact angle of 150
o
is
reached when chitosan is 2,5% wt/v. Nonetheless,
the high concentration of chitosan (3% wt/v)
changes the wettability from superhydrophobic to
hydrophilic (85
o
). This contact angle is in a range of
the chitosan one, which is 70-90
o
(Farris et al.,
2011). This phenomenon corresponds to the
domination of NH
3
+
groups with small nonpolar
structures from beeswax. In addition, there is a lack
of bonding between NH
3
+
groups and fatty acid,
which can conduct the hydrophobicity.
0.25%w/v
128
o
0.5%w/v
134
o
2%w/v
145
o
2.5%w/v
151
o
3%w/v
140
o
0%wt/v
75
o
Contact Angles (deg)
Concentrations (%wt/v)
The Effect of Beeswax and Chitosan Concentrations as Superhydrophobic Coating on Wound Dressing
61
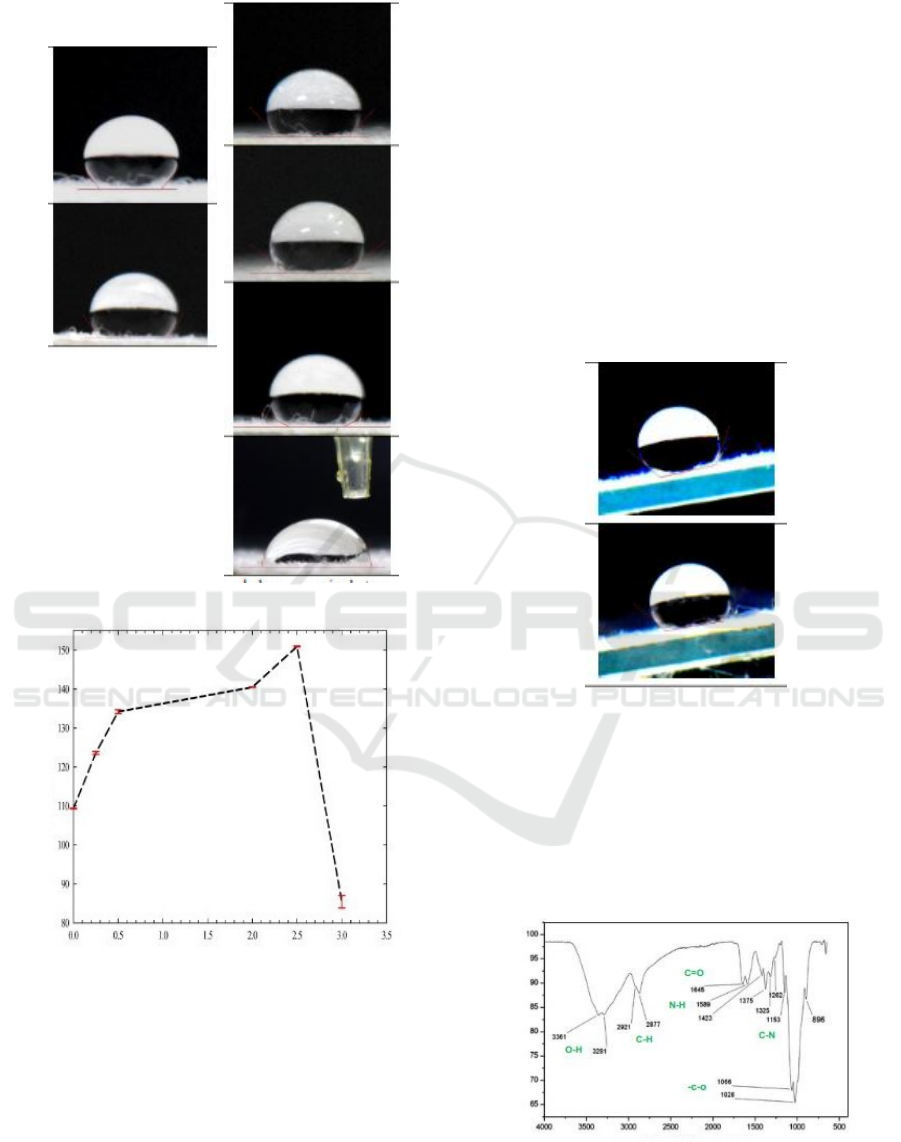
(a)
(b)
Figure.3. (a) The variations of chitosan concentrations and
the fixed beeswax concentration (0.5%w/v) affect the
contact angles. (b) Graphic of contact angles and chitosan
concentrations.
In further, contact angle hysteresis is also
evaluated on the coated substrate. It is conducted by
the tilted-plane method. This test represents an
initial confirmation of whether the drops are pinning
or not. From Fig.4, it is shown that the hysteresis is
small, around one to four degrees. The results potray
that drops can easily slide on the Hypafix. The small
gap between the front and rear angles indicates that
the hysteresis is small. Small hysteresis corresponds
to almost no defects that exist on the surface. The
existence of defects is one of the sources for
capillary force, which will sustain the drop on the
surface. In simple words, small hysteresis relates to
weak capillary force.
In other perspectives, higher contact angle
induces drops to have an almost perfectly round
shape, so there is no big difference between the front
and rear angles. The shape also affects the
movement of the drop. When it has hydrophobic or
superhydrophobic wettability, the drop movement is
dominated by a rolling mechanism than a sliding one
(Wenten et al., 2015).
Figure.4 Contact angle hysteresis of variations beeswax
concentration and fixed chitosan concentration (0.5%w/v)
3.3 Physicochemical Properties of
Superhydrophobic Coating
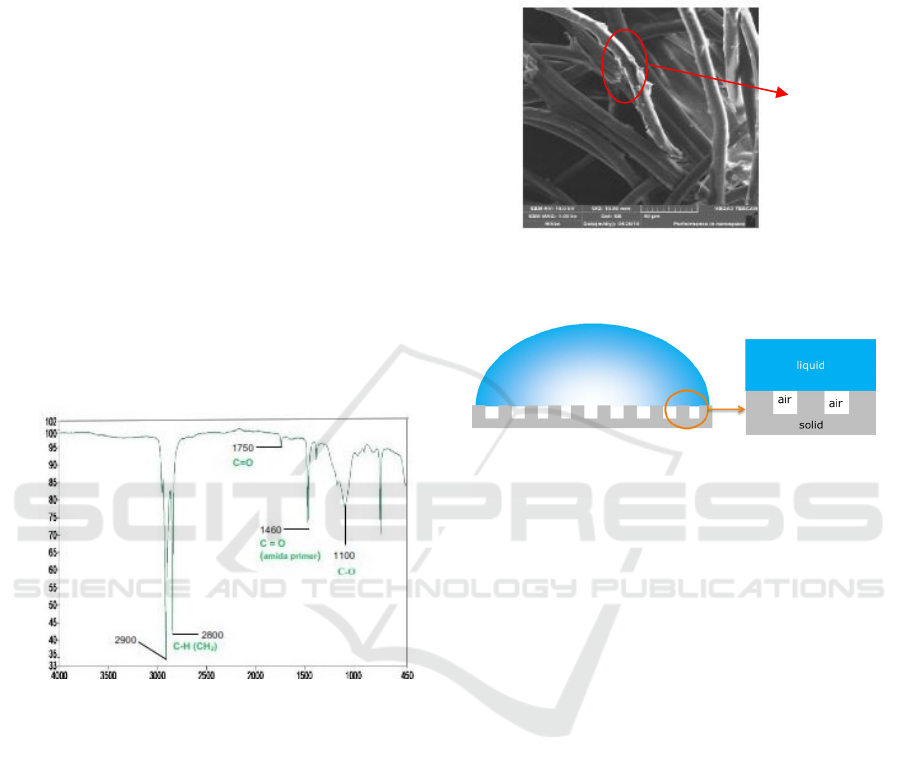
Functional group analysis by FTIR shows that the
coating consists of beeswax and chitosan, as
represented in Figure. 5 and Figure 6
Figure.5. FTIR Spectrum of chitosan (2.5%w/v) and
beeswax (0.5%w/v).
0%w/v
75
o
0%w/v
109
o
0.5%w/v
134
o
2%w/v
140
o
3%w/v
85
o
0.25%w/v beeswax
CAH=4
o
2.5%w/v beeswax
CAH=1.4
o
2.5%wt/v
150
o
Contact Angles (deg)
Concentrations (%wt/v)
Transmittance (%)
Wavelength (cm
-1
)
ICONIT 2019 - International Conference on Industrial Technology
62
(a)
Based on Fig.5, in wavelength of 3361-3291 cm
-
1
, there is vibration from hydrogen bonding between
O-H in chitosan. The alkanes in chitosan that
composed of C-H and N-H are shown in 2921-2877
cm
-1
and 1589 cm
-1
, respectively. The primary amine
and primary amide (C=O in (-NHCOCH
3
) bond) are
also detected in 1153 cm
-1
and 1645 cm
-1
absorbances, respectively. The data of wavelength is
cited from Sigma-Aldrich. The later indicates that
there is a bond between NH3+ groups and beeswax
anions (Wittriansyah et al., 2018).
The functional group in beeswax is shown in
Fig.6. The primary functional groups of beeswax are
indicated as asymmetric C-H (CH
2
), C=O carbonil,
and C-O esther in 2800-2900 cm
-1
, 1750 cm
-1
, and
1100 cm
-1
respectively (Lambert et al., 1998; El-Bisi
et al., 2016; Hromis et al., 2011). The key that shows
if there is a bond between chitosan and beeswax is
primary amide or C=O. This functional group is
found in 1460 cm
-1
absorbance. In those samples, it
can be known that beeswax-chitosan are mixed
uniformly.
Figure.6. FTIR Spectrum of beeswax (2.5%w/v) and
chitosan (0.5%w/v).
To analyze the morphology of coating on the
substrate, the Scanning Electron Microscope is used
as the instrument, and the result is shown in Fig.7
(a). The superhydrophobic coating does not clog the
pores. Hence the wound dressing still has a
breathable characteristic, which keeps air circulation
around the wound. It can be distinguished that the
coated substrate has a film that covers its fibers. The
film contributes to the roughness of the surface. This
roughness contributes to the superhydrophobicity, as
described in Cassie-Baxter Theory that illustrated in
Fig.7 (b). The roughness creates pockets filled by
air. When drop placed on the rough surface, the
interaction between air-substrate is stronger than
drop-substrate interaction. It relates to the higher
surface tension between the drop and substrate.
Hence it makes the drop tends to have a round shape
and has a high contact angle.
Figure.7. (a) Surface morphology of beeswax-chitosan
(0.5%wt/v-2.5%wt/v) in 1000x zoom. (b) Illustration of
the Cassie-Baxter Model
4 CONCLUSION
The superhydrophobic coating on a Hypafix is
produced by a mixture of beeswax and chitosan. The
variations of beeswax and chitosan concentrations in
the mixture affect the wettability properties of the
coating. It can be concluded that beeswax is the
hydrophobic agent that has 151
o
of contact angle in
2.5% wt/v. It is also convinced when beeswax is 3%
wt/v, the superhydrophobicity is maintained in
140.8
o
despite the contact angle decreases from the
early value. Although chitosan also has a close
contact angle in the same concentration (2.5% wt/v),
chitosan is not the primary compound to obtain
superhydrophobicity due to the change of wettability
into hydrophilic (Contact angle=85
o
) when the NH
3
+
groups are dominated (3% wt/v).
Transmittance (%)
Wavelength (cm
-1
)
The fibre
is coated
by
beeswax-
chitosan
mixture
film
(b)
The Effect of Beeswax and Chitosan Concentrations as Superhydrophobic Coating on Wound Dressing
63

ACKNOWLEDGMENT
We acknowledge Lembaga Penelitian dan
Pengabdian Masyarakat (LPPM) Institut Teknologi
Kalimantan, Indonesia, for financial support.
Furthermore, we thank PT Gestrindo Sakti Utama,
Jakarta and PT DKSH Indonesia, Jakarta for
samples characterization.
REFERENCES
Setiyawan, D., 2016, Moist Dressing dan Off-loading
Menggunakan Kruk terhadap Penyembuhan Ulkus
Kaki Diabetik, Tesis, Universitas Muhammadiyah
Yogyakarta, Yogyakarta.
Degreef, H. J., 1998, How to Heal a Wound Fast,
Dermatologic Clinic, Vol. 2, No. 16, pg. 365-375.
Fernandez-Castro, M.; Martin-Gil, B,; Pena-Garcia, I.;
Lopez-Vallecillo, M.; Garcia-Puig, M. E., 2017,
Effectiveness of Semi-permeable Dressings to Treat
Radiation-induced Skin Reactions. A schematic
review, European Journal of Cancer Care, Vol. 6, No.
26.
Wenten, I. G.; Himma, N. F.; Anisah, S.; Prasetya, N.,
2015, Membran Superhidrofobik: Pembuatan,
Karakterisasi, dan Aplikasi, Institut Teknologi
Bandung, Bandung.
Butruk, B. A.; Zietek, P. A.; Ciach, T., 2011, Simple
Method of Fabrication of Hydrophobic Coatings for
Polyurethanes, Central European Journal of
Chemistry, Vol. 9, No.6, pg. 1039-1045.
El-Bisi, M. K.; Ibrahim, H. M.; Rabie, A. M.; Elnagar,
Kh.; Taha, G. M.; El-Alfy, E. A., 2016, Super
Hydrophobic Cotton Fabrics via Green Techniques,
Der Pharma Chemica, Vol.8, No. 19, pg. 57-69.
Naderizadeh, S.; Heredia-Guerrero, J. A.; Caputo G.;
Graselli, S.; Malchiodi, A.; Athanassiou, A.; Bayer, I.
S., 2019, Superhydrophobic Coatings from Beeswax-
in-water emulsion with Latent Heat Storage
Capability, Advanced Materials Interfaces, Vol. 6, No.
5, pg. 1801782.
Lee, S.; Cho, J. S,; Cho, G., 2001, Antimicrobiall and
Blood Repellent Finishes for Cotton and Nonwoven
Fabrics based on Chitosan and Fluoropolymers,
Textile Research Journal, Vol. 69, No.2, pg. 104-112.
Setiani, W.; Sudiarti, T.; Rahmidar, L., 2013, Preparasi
dan Karakterisasi Edible Film dari Polibend Pati
Sukun-Kitosan, Valensi, Vol.3, No.2, pg. 100-109.
Farris, S.; Introzzi, L.; Biagioni, P.; Holz, T.; Schiraldi,
A.; Piergiovanni, L., 2011, Wetting of
BiopolymerCoatings: Contact Angle Kinetics and
Image Analysis Investigation, Langmuir, Germany.
Mohamed, S. A.; El-Sakhawy, M.; Kamel, S. 2017, Water
Resistance and Antimicrobial Improvement of Bagasse
Paper by Microwave Modification with Fatty Acid and
Ag-NPS Nanocomposite, Cellulose Chemistry and
Technology, Vol. 52, No 5-6, pg. 423-431.
Dickinson, E., 2009, Hydrocolloids as Emulsifier and
Emulsion Stabilizers, Food Hydrocolloids, Vol. 23, pg.
1473-1482.
Supeni, G.; Irawan, S., 2012, Pengaruh Penggunaan
Kitosan terhadap Sifat Barrier Edible Film Tapioka
Termodifikasi, Jurnal Kimia Kemasan, Vol. 34, No.1,
pg. 199-206.
Wittriansyah, K.; Handayani, M.; Dirgantara, D., 2018,
Karakterisasi Kitin dan Kitosan Emerita sp. Dari
Pantai Pesisir Widarapayung, Cilacap, Jawa Tengah,
Universitas Diponegoro, Semarang.
Lambert, J. B.; Shurvell, H. F.; Ligtner, D. A.; Cooks, R.
G., 1998, Organic Structural Sprectroscopy, New
Jersey: Prentice Hall.
Hromis, N. M.; Lazic, V. L.; Suput, D. Z.; Popovic, S. Z.;
Tomovic, V. M., 2011, Improvement of water Vapor
Barrier Properties of Chitosan-Collagen Laminated
Casings Using Beeswax, University of Novi Sad, Bul,
Serbia.
ICONIT 2019 - International Conference on Industrial Technology
64
