
Separation of Crude Oil and Its Derivatives Spilled in Seawater by using
Cobalt Ferrite Oxide
Mohammed A. Samba
1
, Ibrahim Ali Amar
2
, Musa Abuadabba
1
, Mohammed A. ALfroji
1
, Zainab M.
Salih
1
and Tomi Erfando
3
1
Department of Oil and Gas , Faculty of Energy and Mining Engineering, Sebha University, Sebha, Libya
2
Department of Chemistry, Faculty of Science, Sebha University, Sebha, Libya
3
Department of Petroleum, Universitas Islam Riau, Pekanbaru, Indonesia
eng.zainabmohammed27@gmail.com, tomierfando@eng.uir.ac.id
Keywords:
Magnetic Cobalt Ferrite Oxide Nanoparticles, Oil Spills, Sea Water.
Abstract:
Oil spills can cause a wide range of impacts in the marine environment and are often portrayed by the media
as ’environmental disasters’ with dire consequences predicted for the survival of marine flora and fauna. The
purpose of this paper is to study the possibility of using spinel oxide (CFO) as an oil absorbent material
with the aim of removing crude oil and its derivatives from aqueous solutions. Spinel oxide from cobalt
ferrite nanoparticles with formula CoFe2O4 (CFO) was prepared by sol-gel method. Functional groups were
also identified on the surface of the oxide using the infrared spectrum (FTIR). In addition, crude oil and its
derivatives were diagnosed using FTIR, and the density and viscosity of crude oil and its derivatives at 15
◦
C temperature. In this study, three samples of seawater were used from different Libyan regions (Gemens
Seawater, Abo Sitta Port, Elbrega Anchorage), and Two samples of crude oil were used from different Libyan
fields (Light, Medium). The samples of crude oil used at three different concentrations (0.01g, 0.03g, 0.05g).
However, the oil removal was calculated for different scenarios as gm / gm and as percentage. The oil removal
capabilities of the prepared absorbent were found to be 10.966 2.3651 g/g to 4.5426 ± 0.113 g/g, 31.8333 ±
5.324 g/g to 7.02053 ± 1.1271, 14.7333 ± 3.1988 g/g to 6.01 ± 0.1287 g/g, 47.1033 ± 6.0222 g/g to 9.2122
± 2.8177, 10.8833 ± 2.1840 g/g to 4.5786 ± 0.1921 g/g, 42.96 ± 1.4046 g/g to 10.5020 ± 1.3172 g/g for
Gemmens Seawater (light oil), Gemmens Seawater (medium oil), Port Abu Sitta (light oil), Port Abu Sitta
(meduim oil), Elbrega Anchorage (light oil) and Elbrega Anchorage (medium oil), respectively. The results
suggest that the prepared magnetic nanoparticles can be used as absorbent materials for removing oil spills
from sea water especially at medium oil.
1 INTRODUCTION
Environmental pollution is the pollution of air, land
and water in many ways. There are several reasons
for environmental pollution, such as from agricul-
ture and industry. Environmental pollution has dras-
tically changed the air, water and terrestrial ecosys-
tems as a result of the industrial revolution in Europe,
North America and China in the 19th century. More-
over, different types of toxic gases and different forms
of carbon components were produced from factories,
transport, and energy sectors has resulted in different
changes in the global climate and weather patterns,
and become a source of contamination of land, as well
as the ocean environment where the average temper-
ature and acidity are increasing. In addition, many
other chemicals like fertilizers used in the agricultural
industry also contribute to the pollution of the seas
over vast areas (Fartoosi and M., 2013).
Oil spills can have devastating effects on water-
ways and oceans. In the oil it is the polycyclic aro-
matic hydrocarbons (PAHs) that cause most of the
toxicity for human life, but the physical nature of oil,
i.e. the stickiness is a major problem for a number of
organisms such as birds. Spills of oil has a numerous
negative impacts both short and long term, resulting
in economic and financial losses. Also, the recover-
ing and clean-up processes are very costly; see for
example cases such as the clean-up from the Exxon
Valdes or the Deep Water Horizon (Fartoosi and M.,
2013). Oil spills could be removed through many
methods such as mechanical, chemical and treatment
Samba, M., Amar, I., Abuadabba, M., Alfroji, M., Salih, Z. and Erfando, T.
Separation of Crude Oil and Its Derivatives Spilled in Seawater by using Cobalt Ferrite Oxide.
DOI: 10.5220/0009146901750181
In Proceedings of the Second International Conference on Science, Engineering and Technology (ICoSET 2019), pages 175-181
ISBN: 978-989-758-463-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
175

by burning in situ, this study will include a part of the
chemical methods spinel oxide nanoparticles (mag-
netic spinal compounds).
1.1 Magnetic Spinel Compounds
The mixed transition elements of the general formula
AB2O4 are called spinel’s. These oxides take their
name from spinel metal (MgAl2O4). A is a binary
ion (Fe
2
+, Co
2
+, Ni
2
+, Zn
2
+, Mg
2
+, etc.), while B
is a trivalent ion (Fe
3
+, Co
3
+, Cr
3
+, Al
3
+, Mn
3
+, etc. ) (Smart, ). Spinel oxides are among the
most important magnetic nanomaterial’s. Spinel fer-
rite, SF Magnetic nanoparticles are spinel oxides con-
taining tri-iron ions. These oxides have the general
formula M
2
+ Fe
3
+ O
4
(Where M
2
+ represents Mn
2
+, Fe
2
+, Co
2
+, Ni
2
+, Zn
2
+, Mg
2
+ etc.). These
oxides have distinct chemical and physical properties
(Reddy, 2016). Excellent magnetic properties, Large
surface area, its surface has a large number of effec-
tive sites, high chemical stability, easy to prepare and
convert to the desired shape (Smart, ; Gomez-Pastora
et al., ).
Spinel oxides has wide applications in several
fields including: gas sensors, magnetic devices, wa-
ter purification, medicine, catalysts, recharging bat-
teries and ammonia production by electric stimulation
(Amar, 2014), as shown in figure 1.
In the field of water treatment, spinel nano-
magnetic nanoparticles on the list of materials can
be used as absorbent materials, because it can be re-
moved quickly and easily from the solution after ab-
sorption using an external magnetic field, after sepa-
rating the pollutants can be removed and reused sev-
eral times, Water Purification.
2 MATERIALS AND CHEMICALS
CoFe
2
O
4
were used in this study. Seawater three sam-
ples were used after routine testing and distilled water.
Light and Medium crude oil were also used with (den-
sity: 0.8245, API: 40, viscosity: 5.6136 and Boris
3.5915 @ 25
◦
C and @ 37.5
◦
C, Sp.Gr @ 60/60Fo
:0.8249) for light. While the (density: 0.8368, API:
37.5, viscosity: 19.5970 and Boris 9.7102 @ 25
◦
C and @ 37.5
◦
C, Sp.Gr @ 60/60 Fo:0.8372) for
medium.
2.1 Cobalt Ferrite Oxide Nanoparticle
Particles (CoFe
2
O
4
)
The magnetic spinal Cobalt ferrite oxide powder was
prepared 10 gm from magnetic spinal Nanoparticle
Figure 1: Some applications of spinel ferrite oxides (Amar,
2014)
formula CFO )COFe
2
O
4
(by Sol-gel method (Amar
et al., 2018), and The required quantities of cobalt
nitrate (12.4041g) and iron nitrate (34.4369g) were
weighed, Then add it in a small amount of distilled
water. Then add citric acid in amount of (36.8486g)
and EDTA in amount of (37.34g) as complication fac-
tors, the ammonia solution (NH
3
.H
2
O) was added to
pH control to 6, The solution was then evaporated us-
ing an electric heater with the solution moving contin-
uously by a magnetic mold to distribute heat. And by
continuously heating and stirring solution (mixture)
to thick black gel, the magnetic stirrer was then re-
moved and the gel left on the electric burner com-
pletely burned and turned into a solid component
(ash), The component was milled solid obtained and
placed in ceramic seals and burned in the air in the
furnace at 600
◦
C for 2 hours to remove the remaining
organic compounds and obtain a pure phase of pow-
der (Cobalt ferrite oxide), which will later be used as a
maze material to remove the blue methylene dye from
its solutions water. Figure 2 Shows the procures to
prepare the spinal oxide (Scheffe et al., 2011; Scheffe
et al., 2013).
2.2 Sea Water and Crude Oil
Three samples of seawater were obtained from dif-
ferent parts of Libya, Tripoli, Benghazi and Al Brega
as shown in the figure 3. The parameters of seawa-
ter were calculated at the Faculty of Science Univer-
sity of Sebha, as shown in Table 1. Two samples of
crude oil were collected from different fields of Libya
(Light, Medium) As shown in the figure 4, Crude oil
properties were calculated at the Tripoli Petroleum
Research Center as shown in table 2.
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
176
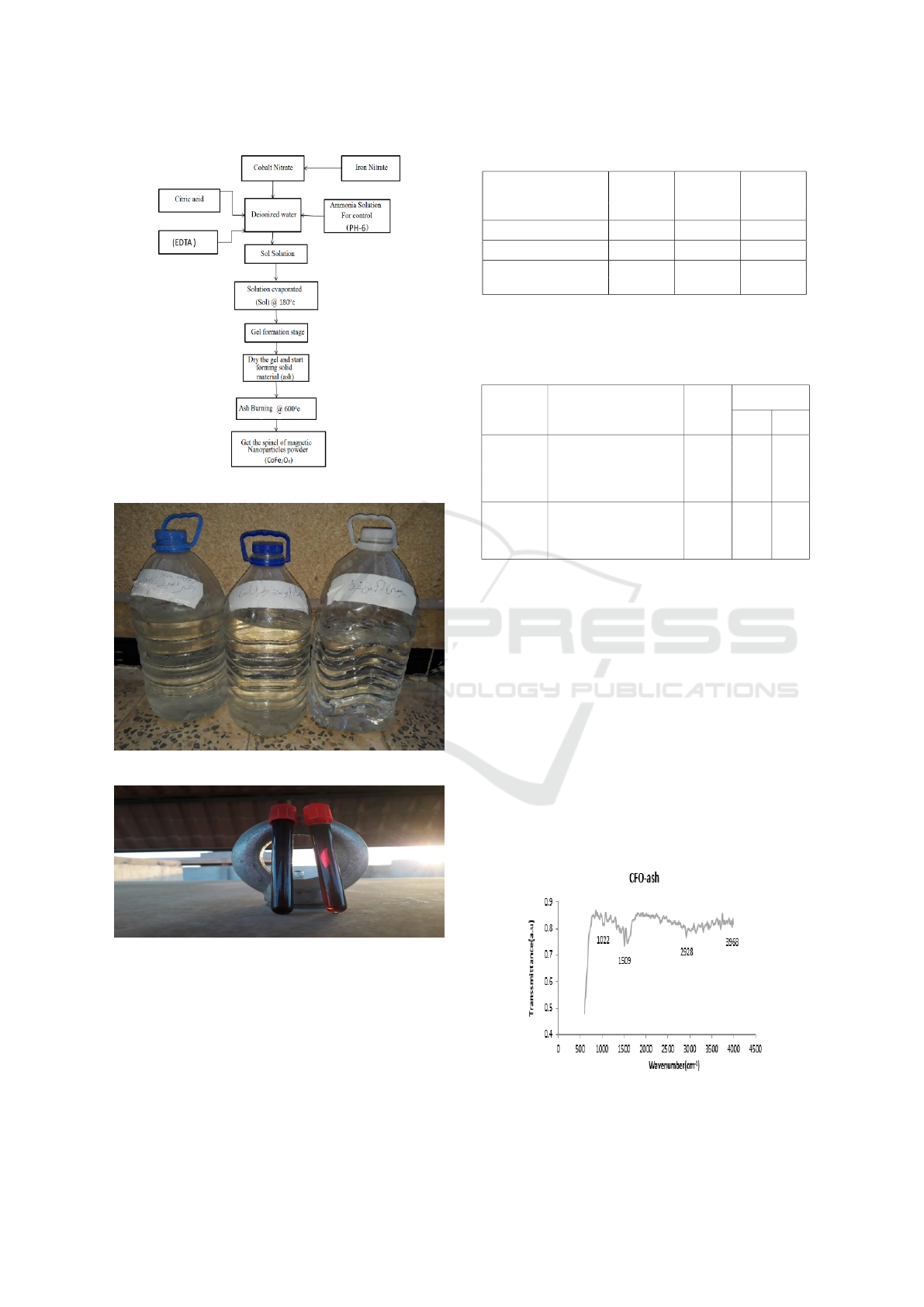
Figure 2: Steps of Preparation Spinal oxide
Figure 3: Shows seawater samples that used in this study
Figure 4: Shows oil samples that used in this study
3 RESULTS AND DISCUSSION
3.1 FTIR Result
Functional groups on the surface of the spinel oxide
prepared by the sol-gel method and then identified us-
ing the infrared absorption spectrum (FTIR). The car-
Table 1: The Properties of seawater for three samples
Type
Marsa Sea
Gmines
Mina
Abo-seta
al-Briga /Benghazi /Tripoli
Conductivity (mc/cm) 183.9 186.7 180.7
pH 7.34 7.9 7.52
Salinity
1222810 1314210 1132110
(ppm)
Where:
MC/CM = Milli Cemence/Centimeter
Ppm = Pond per Million
Table 2: The Properties of Crude Oil.
Test Method TestDescription Unit
Result
X- field X-field
ASTM D 5002 Density @ 15
◦
CSp.Gr 60/60FAPI g/cc////
0.8368 0.8245
0.8372 0.8249
37.5 40
ASTM D 445
K.Viscosity@25
◦
C m 19.597 5.6136
K.Viscosity@
37.8
◦
C
m
2
/s 9.7102 3.5915
Where:
ASTM = American Society for Testing And Material.
K.Viscocity = Kinematic Viscocity Sp.Gr = Specific
Gravity
bon nanotube (CFO) oxide after burning the ash com-
pounds in preparation in air at 600
◦
C for two hours.
Figure 5 shows the FTTR results for Nanoparticles,
it is clearly seen that there are bundles of the termi-
nals at 3968 cm
-1
and 2928 cm
-1
; these packets can
be attributed to the Co-O and Fe-O bonds, respec-
tively. These specialty packs are characteristic of all
spinel oxides. Figure 6 and figure 7 have shown the
FTTR results for different oil type, where the range of
wavenumber from 600 to 4000 cm
-1
.
Figure 5: FTIR results for nanoparticles
Separation of Crude Oil and Its Derivatives Spilled in Seawater by using Cobalt Ferrite Oxide
177
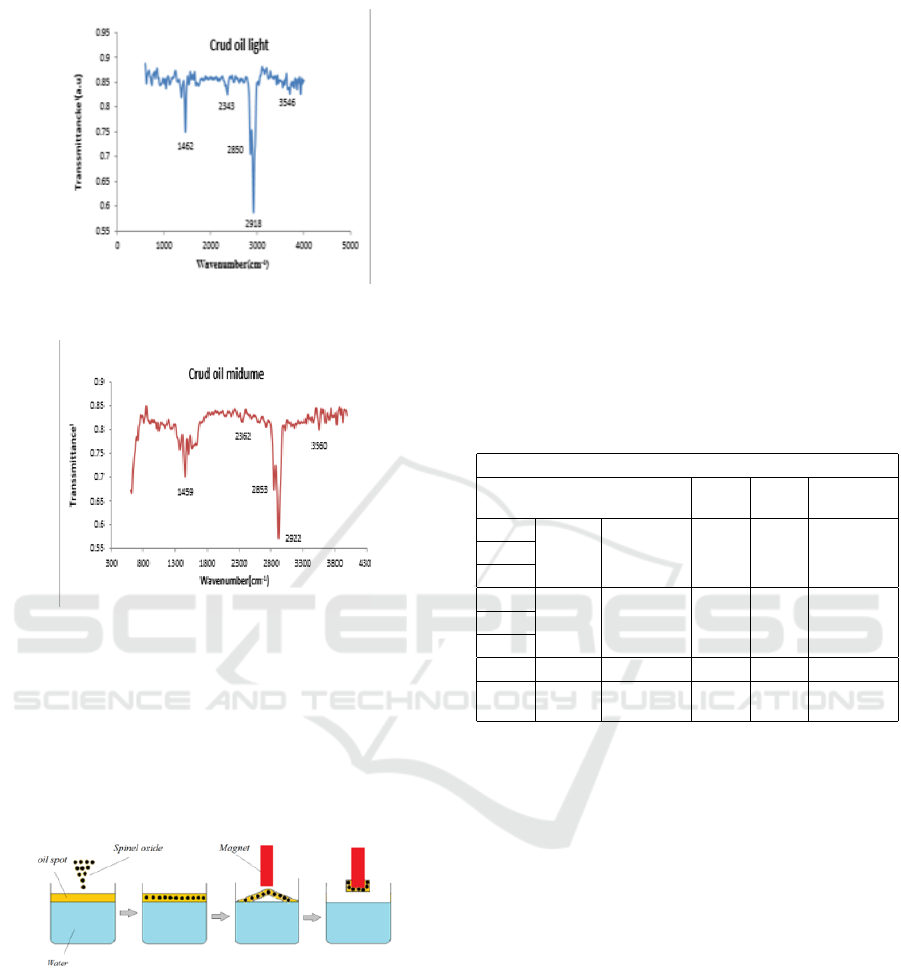
Figure 6: FTIR Results for light oil.
Figure 7: FTIR Results for medium oil.
3.2 Results of Oil Removal as
gram/gram g/g
The technique of remove the oil from the sea water a
by using magnetic rod has shown in the figure 8.
Figure 8: Shows the step of removal oil spot from water
surface (Amar et al., 2019)
The following equation for calculating the oil re-
moved:
OR = (m2 − m1)/m1 (1)
Where:
OR=Oil Removal (gm/gm).
m1= Concentration of the spinal (gm).
m2= The weight of the spinal and oil (gm).
3.2.1 Gemmens Seawater
Figure 9,10 and table 3 display the gravimetric oil re-
moval (OR, g/g) or the oil absorption capacity of the
tested oily samples (light and medium) of Gemmens
Seawater as a function of absorbent amount. As can
be seen, in all cases the gravimetric oil removal of
Gemmens Seawater decrease with the increase in the
amount of the adsorbent from 0.01 to 0.05 g. In the
case of light oil (Figure 9), the OR decreased from
10.966 ± 2.3651 g/g to 4.5426 ± 0.113 g/g as the
amount of absorbent material. For the medium oil, the
OR was about 31.8333 ± 5.324 g/g at the absorbent
amount of 0.01 g and reached a value of 7.02053 ±
1.1271 g/g when the absorbent amount increased to
0.05 g (Figure 10).
Table 3: The Properties of Crude Oil.
Gemmens
Meduim Light
O.R
Concentrate
Sd O.R
Concentrate
Sd
31.8333 0.01 2.36 10.966 0.01
5.324
1.5565 13.8388 0.03 0.3967 5.6955 0.03
1.1271 7.2053 0.05 0.113 4.5426 0.05
Where:
OR=Oil Removal.
Sd=Standard Devition.
3.2.2 Port Abu Sitta
Figure 11,12 and table 4 display the gravimetric oil
removal (OR, g/g) or the oil absorption capacity of
the tested oily samples (light and medium) of Port
Abu Sitta Seawater as a function of absorbent amount.
As can be seen, not all cases the gravimetric oil re-
moval of Abu Sitta Seawater decrease with the in-
crease in the amount of the adsorbent from 0.01 to
0.05 g. In the case of light oil (Figure 11), the OR de-
creased from 14.7333 ± 3.1988 g/g to 6.01 ± 0.1287
g/g as the amount of absorbent material. For the
medium oil, the OR was about 47.1033 ± 6.0222 g/g
at the absorbent amount of 0.01 g and reached a value
of 9.2122 ± 2.8177 when the absorbent amount in-
creased to 0.03 g, while 10.0593 ± 0.8987 g/g when
the absorbent amount increased to 0.05 g (Figure 12).
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
178
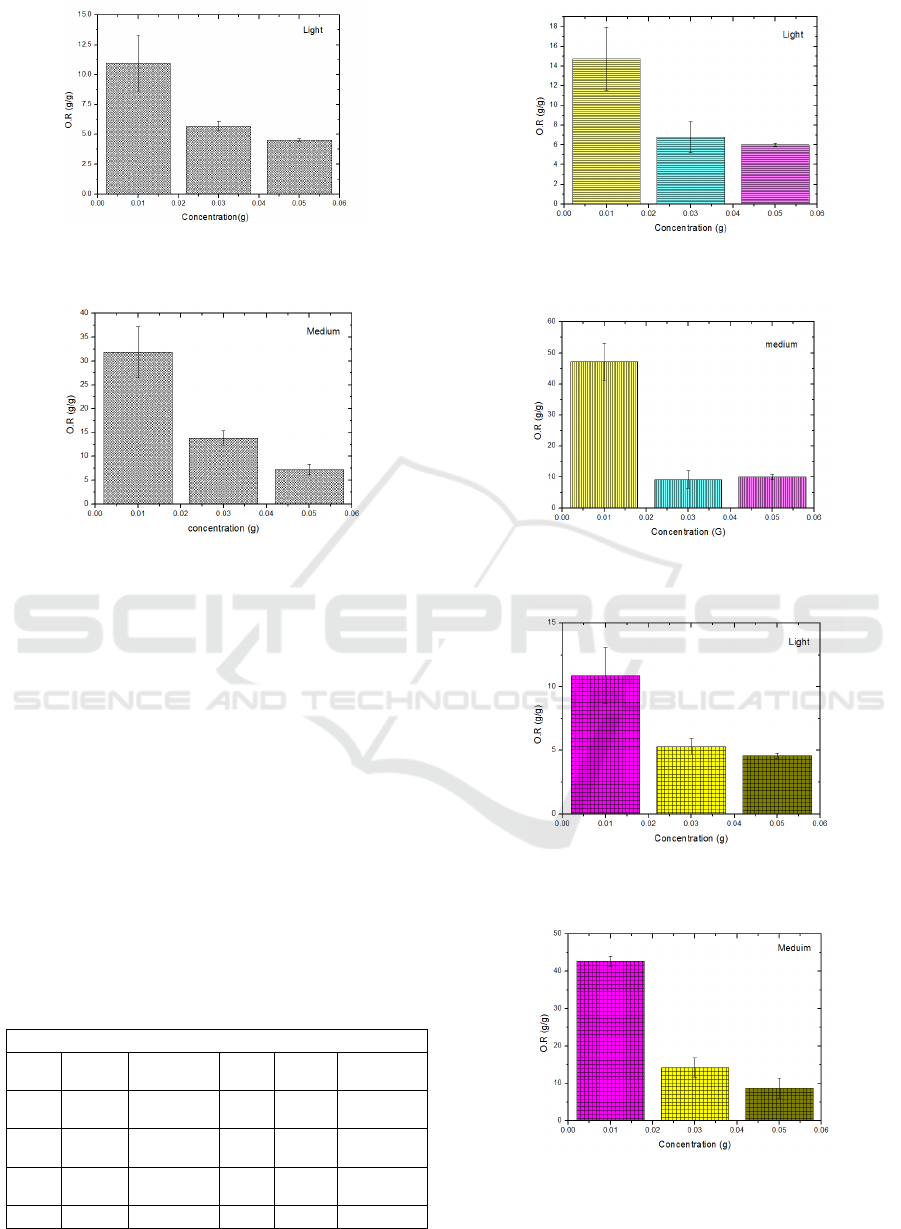
Figure 9: Shows The Crude Oil (Light) Concentration, Oil
Removal Gemmens
Figure 10: Shows the Crude Oil (Medium) Concentration,
Oil Removal Gemmens
3.2.3 Elbrega Anchorage
Figure 13,14 and table 4 display the gravimetric oil re-
moval (OR, g/g) or the oil absorption capacity of the
tested oily samples (light and medium) of Elbrega An-
chorage Seawater as a function of absorbent amount.
As can be seen, in all cases the gravimetric oil re-
moval of Elbrega Anchorage Seawater decrease with
the increase in the amount of the adsorbent from 0.01
to 0.05 g. In the case of light oil (Figure 13), the OR
decreased from 10.8833 ± 2.1840 g/g to 4.5786 ±
0.1921 g/g as the amount of absorbent material. For
the medium oil, the OR was about 42.96 ± 1.4046 g/g
at the absorbent amount of 0.01 g and reached a value
of 10.5020 ± 1.3172 g/g when the absorbent amount
increased to 0.05 g (Figure 14).
Table 4: The Properties of Crude Oil.
PORT ABU SITTA
Medium Light
Sd O.R Concentrate Sd O.R Concentrate
6.0222 47.1033 0.01 3.1988 14.7333 0.01
2.8177 9.2122 0.03 1.598 6.8199 0.03
0.8987 10.0593 0.05 0.1287 6.01 0.05
Figure 11: Shows the Crude Oil (Light) Concentration, Oil
Removal Port Abu sitta
Figure 12: Shows the crude oil (medium) concentration, oil
removal port Abu sitta
Figure 13: Shows the crude oil (light) concentration, oil
removal Elbrega Anchorage
Figure 14: Shows the crude oil (medium) concentration, oil
removal Elbrega Anchorage
Separation of Crude Oil and Its Derivatives Spilled in Seawater by using Cobalt Ferrite Oxide
179
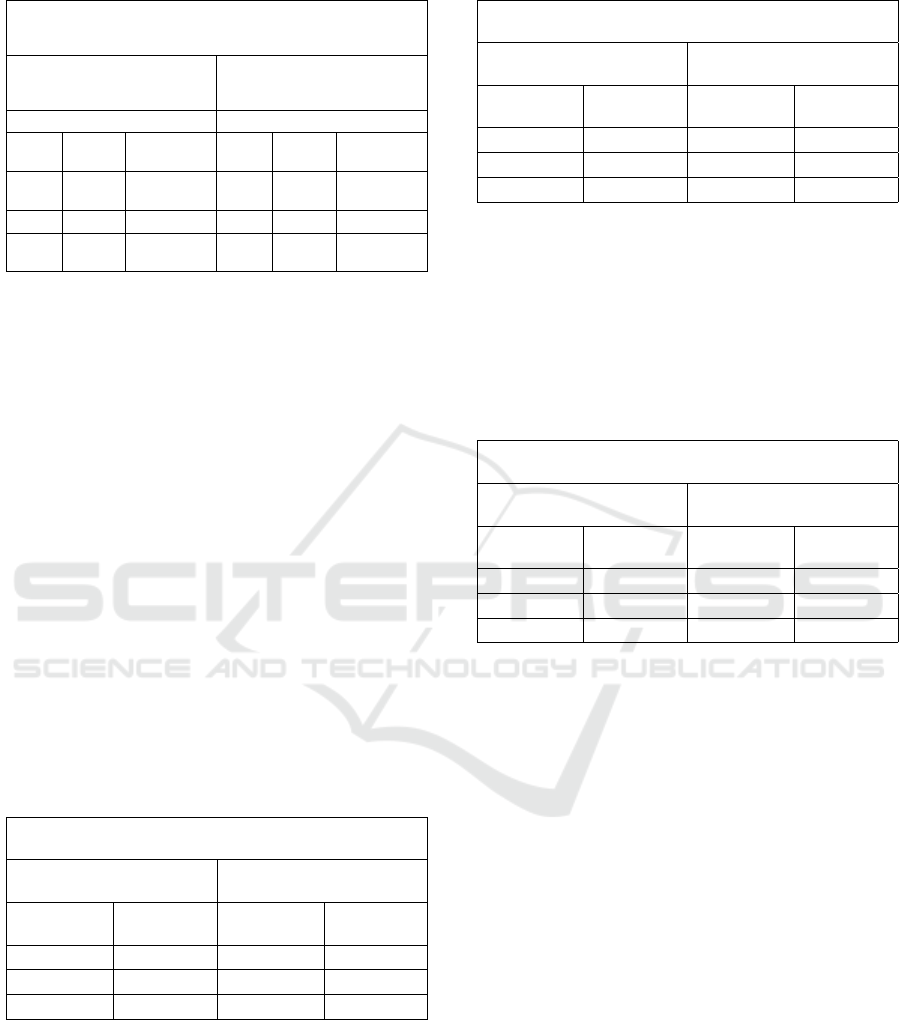
Table 5: The Properties of Crude Oil.
Elbrega Anchorage
Medium Light
Sd O.R Concentrate Sd O.R Concentrate
1.4046 42.69 0.01 2.184 10.8833 0.01
2.5788 14.2086 0.03 0.6217 5.3132 0.03
1.3172 10.502 0.05 0.1921 4.5786 0.05
3.3 Results of Oil Removal as
Percentage
The following equation for calculating the percent-
age:
Remaining = ((Woil +Powder)−W removal)/(Woil +Powder)
(2)
OilRemovalPercentage = (1 − Remaining) ∗ 100 (3)
3.3.1 Gemmens Seawater
The highest percentage of oil removal was (52.73%)
at the powder concentration (0.05gm) during the light
oil, when using the medium oil, the highest oil re-
moval percentage was (79.43%) at the powder con-
centration(0.03gm) as shown in the table 6.
Table 6: Shows the Oil Removal Percentage of Gemmens
Seawater
Gemmens
Light Medium
Concentration Percentage % Concentration Percentage %
0.01 24.65 0.01 60.92
0.03 33.74 0.03 79.43
0.05 52.73 0.05 65.09
3.3.2 Port Abu Sitta
When we use the light oil was the highest removed
(66.69%), It was when the powder concentration
(0.05gm), When using the medium oil was the highest
removal rate (91.1%), It was when the powder con-
centration (0.05gm) as shown in the table 7.
Table 7: Shows the oil removal percentage of Abu Sitta port
Port Abu Sitta
Light Medium
Concentration Percentage % Concentration Percentage %
0.01 32.41 0.01 89.25
0.03 46.41 0.03 54.81
0.05 66.69 0.05 91.1
3.3.3 Elbrega Anchorage
The light oil was the highest removed about (53.08%),
It was when the powder concentration (0.05gm),
When using the medium oil was the highest removal
rate (93.1%), It was when the powder concentration
(0.05 gm) as shown in the table 8.
Table 8: Shows the oil removal percentage of Abu Sitta port
Port Abu Sitta
Light Medium
Concentration Percentage % Concentration Percentage %
0.01 25.17 0.01 82.79
0.03 37.47 0.03 80.92
0.05 53.08 0.05 93.1
4 CONCLUSION
The properties of iron oxide were studied and func-
tional groups were identified using the infrared spec-
trum. Two types of oil samples (Light and Medium)
were used as water pollutants model. Within the ab-
sorbent amount of 0.01 to 0.05 g, the gravimetric
oil removal capabilities were between the 24.5% to
93.1%. The obtained results suggest that Cobalt Fer-
rite Oxide Nanoparticle might be promising absorbent
materials and can be used for oil-spill cleanup from
Sea water specially for medium oil.
• The material must be milled enough to avoid
falling into the bottom of the test.
• High-density raw materials must be heated when
aggregated in cold temperatures.
• Apply experiments in large vessels for easy han-
dling with magnets and to contribute to the suc-
cess of the experiment.
• A medium-sized absorbent should be used for
easy handling with the addition of oil.
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
180

REFERENCES
Amar, I. A. (2014). et al (2014). Electrochemical synthesis
of ammonia from N 2 and H 2 O based on (Li, Na, K)
2 CO 3Ce 0. 8 Gd 0. 18 Ca 0, 8(18).
Amar, I. A. et al. (2018). Synthesis and Characterization of
Magnetic CoFe1. 9Cr0.
Amar, I. A. et al. (2019). Oil spill removal from wa-
ter by absorption on zinc-doped cobalt ferrite mag-
netic nanoparticles. Advanced Journal of Chemistry-
Section A (Theoretical, Engineering and Applied
Chemistry), pages 266–385):.
Fartoosi, A. and M., F. (2013). The impact of maritime
oil pollution in the marine environment: case study
of maritime oil pollution in the navigational channel
of Shatt Al-Arab.
Gomez-Pastora, J. et al. (2014).
Reddy, D. H. K. (2016). Spinel ferrite magnetic adsor-
bents: alternative future materials for water purifica-
tion? Coordination Chemistry Reviews.
Scheffe, J. R., Allendorf, M. D., Coker, E. N., Jacobs,
B. W., McDaniel, A. H., and Weimer, A. W. (2011).
Hydrogen production via chemical looping redox cy-
cles using atomic layer deposition-synthesized iron
oxide and cobalt ferrites. Chemistry of Materials,
23(8):2030–2038.
Scheffe, J. R., McDaniel, A. H., Allendorf, M. D., and
Weimer, A. W. (2013). Kinetics and mechanism of
solar-thermochemical h 2 production by oxidation of
a cobalt ferrite–zirconia composite. Energy & Envi-
ronmental Science, 6(3):963–973.
Smart, L. E. Solid state chemistry: an introduction. Third
Edition.
Separation of Crude Oil and Its Derivatives Spilled in Seawater by using Cobalt Ferrite Oxide
181
