
Antibacterial and Characterization of Secondary Metabolite
Compound from Ethyl Acetate and Ethanol Fraction of Leaves
Moringa oliefera L
Siti Rofida
Pharmacy Departement, Faculty of Health Science, University of Muhammadiyah Malang, Jalan Bendungan Sutami 188A,
Malang 65145, East Java Indonesia.
Keywords: Fractionation, Moringa oleifera leaves, Secondary metabolite compounds, Antibacterial.
Abstract: The microorganisms that cause infections can mutate due to excessive antibiotic exposure. One of the new
drug search strategies is through the exploration of active ingredients derived from plants that have been used
empirically by the community. Moringa oleifera L leaf is a plant that has been used and has been shown to
have antibacterial, antifungal, analgesic, and antihypertensive activities. Moringa oleifera L leaves contain
secondary metabolites such as alkaloids, tannins, saponins, flavonoids, and phenols. The purpose of this study
was to obtain active ingredients from the leaves of Moringa oleifera L which will be used as a Standardized
Herbal Medicine product in the treatment of infectious cases. In order to get the active ingredient as an
antibacterial, multilevel extraction is carried out using different polarity solvents, so that a fraction containing
nonpolar, semipolar and polar compounds will be obtained. Antimicrobial potential will be tested on each
fraction using the disk diffusion method. The results of identification of the compounds in the ethyl acetate
fraction show the class of compounds Flavonoids, Terpenoids, Polyphenols and Anthraquinone while in the
ethanol fraction Moringa oleifera L. leaves show the compounds of the compounds Alkaloids, Flavonoids,
Terpenoids, Polyphenols, and Saponin. Antimicrobial activity is shown in the ethyl acetate and ethanol
fraction in both Staphylococcus aureus and Escherichia coli.
1 INTRODUCTION
Infection is a pathological condition caused by
microorganisms such as bacteria, viruses, fungi, and
protozoa, and can occur in the community or in
hospitals. Patients who are being treated at the
hospital, have a greater risk of contracting the
infection than outside the hospital. This can occur as
a result of interactions between patients,
environments, and microbes showed that 10 rooms
out of 16 inpatient rooms in the "X" hospital in
Semarang City had airborne germ exceeding the total
threshold of germs in the inpatient room (Wikansari,
2012). Infections that occur in hospitals and attack
patients who are in the process of treatment are
known as nosocomial infections. The prevalence of
nosocomial infections in Indonesia is 7.1%
(Wikansari, 2012). Various attempts have been made
by the hospital in dealing with nosocomial infections
namely by washing hands before and after contact
with patients; use personal protective equipment such
as gloves, masks, and other personal protective
equipment; decontaminate equipment after use in
service; sharp tool management; medical and non-
medical waste management.
Handling in cases of infection is antibiotic
therapy. But now some antibiotics are no longer able
to deal with cases of infection because they are caused
by antibiotic resistance. This antibiotic resistance
occurs because of the use of antibiotics freely, so that
microorganisms become more resistant to antibiotics.
The resistance of microorganisms to antibiotics
occurs because they are too often exposed to
antibiotics so that microorganisms undergo mutations
to form a biofilms layer so that the cell walls are
thicker and cannot be penetrated by antibiotics. This
mechanism is a self-defense mechanism for
microorganisms to be able to survive (Brooks et al.,
2013). The highest resistance found in antibiotics
penicillin and cephalosporin first generation (Yacob
et al., 2011).
This antibiotic resistance if left untreated can
cause cases of infection to become uncontrollable.
Rofida, S.
Antibacterial and Characterization of Secondary Metabolite Compound from Ethyl Acetate and Ethanol Fraction of Leaves Moringa oliefera L.
DOI: 10.5220/0009141002330239
In Proceedings of the 2nd Health Science International Conference (HSIC 2019), pages 233-239
ISBN: 978-989-758-462-6
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
233

The increase in incidence will be very rapid because
infection is a contagious disease. Efforts made to
prevent antibiotic resistance are through the
establishment of antibiotic use policies only in
diseases which according to laboratory data have
indeed been proven to be due to microorganisms.
Another effort undertaken is to explore new
medicines with plant sources that have been proven
empirically efficacious as antimicrobials. Secondary
metabolite compounds that have been shown to have
antimicrobial properties are a group of phenolic
compounds such as simple phenols, phenolic acids,
quinones, flavones, flavonoids, tannins, coumarin;
terpenoid compounds and essential oils; and alkaloids
(Luqman et al., 2012; Akinyeye, Solanke and
Adebiyi, 2014; Gyawali and Ibrahim, 2014; Minaiyan
et al., 2014).
One of the plants that has been empirically
proven as an antibacterial is Moringa oleifera L. This
plant contains alkaloids, tannins, saponins,
flavonoids, and phenols (Oluduro, 2012). Moringa
oleifera leaf extract has antimicrobial activity against
bacteria Klebsiella pneumonia, Escherichia coli,
Staphylococcus aureus, and Streptococcus
pneumonia (Kalpana, Moorthi and Kumara, 2013).
The study was conducted using agar diffusion
methods and extracts used 200-800mg / ml. The
solvent used is chloroform, petrolatum ether, ethanol,
and water. From these studies, the results showed that
all extracts showed antimicrobial activity. At a
concentration of 800mg the average inhibition zone
showed Klebsiella pneumonia 9.3 ± 0.46, Escherichia
coli 11.0 ± 0.00, Staphylococcus aureus 13.0 ± 0.00,
and Streptococcus pneumonia 7.0 ± 0.81. The ethanol
extract showed that the maximum inhibition zone was
S. aureus and the smallest inhibitory zone water
extract was in Streptococcus pneumonia. For this
study, the positive control used was tetracycline 10
mcg.
Based on the results of previous studies show that
the leaves of Moringa oleifera L. have the potential
as raw materials for standardized herbal medicines as
antimicrobials. Raw materials for standardized herbal
medicines can be in the form of extracts or fractions
resulting from the separation of plant secondary
metabolite compounds (Sarker, Latif and Gray,
2006). Based on research conducted by Rofida, et al
(2017) that the fractionation process of Garcinia
mangostan Linn extract can increase its cytotoxicity
effect on T47D cell culture. This shows that there is
an accumulation of active compounds in the
extraction fraction.
In order to obtain active ingredients from Moringa
oleifera L. leaves, multilevel extraction will be
carried out using multilevel extraction techniques
using solvents that have different polarities so that
non-polar compounds, semipolar compounds, and
polar compounds will be obtained. Multilevel
extraction using different polarity solvents will
separate secondary metabolites based on their
solubility. Groups of base form alkaloids, free
flavonoids, and free terpenoids will be more easily
extracted with nonpolar to semipolar solvents.
Whereas the alkaloids in the form of salts, flavonoids
glycosides, terpenoid glycosides, and polyphenols are
more easily extracted in polar solvents (Sarker et al.,
2006). The fraction of the results of the separation
will be tested for antimicrobial potential, especially in
gram-positive bacteria (Staphylococcus aureus) and
gram-negative (Escherichia Coli) in vitro by disc
diffusion method. Furthermore, the active ingredient
of Moringa oleifera L. leaves which has potential as
an antimicrobial will be characterized by secondary
metabolite compounds by the TLC method.
2 METHODS
Material
Moringa oleifera leaves L., Staphylococcus aureus
Escherichia Coli, Silica Gel TLC Plate GF 254, Ethyl
acetate, Ethanol, Mueller Hinton Agar medium.
Fractionation
Moringa oleifera L. leaf powder was extracted
stratified with hexane, ethyl acetate, and ethanol as
solvent respectively with maceration technique. The
ingredients are soaked in hexane solvent for 24 hours,
then filtered and separated the filtrate. The residue is
given the same treatment. This treatment is repeated
until the filtrate does not show stains on the silica
plate. The filtrate obtained was concentrated using a
rotary evaporator at 50°C until a thick extract was
obtained. Each viscous fraction obtained was dried in
an oven at 40°C. Each thick fraction ready for use is
stored in a refrigerator at 5-8°C.
Preparation Sample
The test solution to be used was made by weighing
each fraction of Moringa oleifera L. as much as 50
mg, 25 mg, 12.5 mg dissolved in 0.1 ml of 1% DMSO
then added with sterile aquadest to 1 ml to obtain the
concentration of the test solution in the amount of 50
ml / mL; 25 ml / mL; 12.5 mg / mL
Antibacterial Activity by Disk Diffusion Method
The test was carried out by filling sterilized Petri
dishes with nutrient media to as much as 20 mL and
HSIC 2019 - The Health Science International Conference
234

waiting for it to harden. Then the bacteria is applied,
evenly with the streak plate method. The media used
for bacterial culture is sterile aquadest that has been
standardized with McFarland's standard (106 CFU /
ml) (McFarland, 1907). The hexane, ethyl acetate and
ethanol fractions of M. oleifera leaves were weighed
50mg each dissolved in 1 ml of solvent (according to
the solvent's flavor) and then bottled on the TLC plate
as much as 5 µl. The hexane fraction was eluted with
n-hexane: ethyl acetate: formic acid (6.5: 3.5: 3
drops) mobile phase, the ethyl acetate fraction used
the n-hexane: ethyl acetate (4: 6) mobile phase system
and ethanol fraction uses a mobile phase system of n-
hexane: ethyl acetate: methanol (0.5: 4: 0.5) plus 1
drop of formic acid. The spot stains that are covered
are cut and sterilized for 30 minutes using UV light in
LAF (Laminar Air Flow). The TLC plate is then
planted in a petri dish that contains bacterial cultures.
Petri dishes are incubated for 24 hours at 37oC. Then
the inhibition zone is formed. Antibacterial testing
was replicated three times. As a positive control,
erythromycin 15 µg / disk was used. As a negative
control, TLC plates were used which were incubated
without any test material spills.
Characterization of Secondary Metabolite
Compounds with Thin Layer Chromatography
Methods
Each extract produced was carried out by thin-layer
chromatography test using silica gel F254 stationary
phase and various eluent mobile phases. The TLC
profile was observed with UV lamps 254 and 365. To
find out the class of compounds, the TLC results were
derivatized with dragendorf solvent, anisaldehyde-
sulfuric acid, FeCl3, KOH, and 10% H2SO4.
3 RESULTS AND DISCUSSION
From the concentration process, it was obtained that
the ethyl acetate fraction of M. oleifera leaves was as
thick as 7.83 g yield of the fraction produced was
3.132%. The yield of ethanol fraction was 42.65 g,
the yield of the fraction extracted from M. oleifera
leaves was 17.06%. Antibacterial activity of M.
oleifera L. leaves
fractionation against Staphylococcus aureus and
Escherichia Coli bacteria can be seen in Table 1. The
identification test carried out on M. oleifera leaf ethyl
acetate fraction using thin-layer chromatography
(TLC) method showed that there were no alkaloid
compounds. The results of the TLC test can be seen
in Figure 1. Based on table 1, the ethl acetate fraction
and ethanol fraction showed that the compound with
Rf 0.9 showed the highest antibacterial activity
against Staphylococcus aureus Escherichia Coli.
Gram-negative bacteria have a way to protect their
cell membranes from penetrating antibacterial agents,
because they have a unique outer membrane,
relatively thinner peptidoglycan walls, and
periplasmic space between the cell wall and
membrane. This outer membrane structure contains
Lipopolysaccharides (LPS) or endotoxins, a complex
structure consisting of Lipid A, short chains of sugar
and long chains of carbohydrates called O-antigens.
O antigens and polysaccharides contained in bacterial
outer membranes play a role in preventing the
penetration of hydrophobic compounds , such as
anthraquinone compounds, into the cell membrane,
while the penetration of hydrophilic compounds, such
as phenol and tannin compounds, into the cell
membrane is prevented by the lipid properties they
have (Brooks et al., 2013).
The results of identification by TLC method
against the ethyl acetate fraction of M.oleifera leaf
showed that there was a class of terpenoids
compounds (Figure 1), flavonoids compounds
(Figure 2), polyphenol compounds (Figure 3),
anthraquinone compounds (Figure 4). The results of
identification of compounds by TLC technique on
spot color observation both visually and irradiated by
UV 254 nm and 365 nm, obtained Rf value presented
in Table 2. In previous studies it was found that the
ethyl acetate fraction of M. oleifera leaves contained
chemical compounds such as alkaloids, flavonoids,
saponins, tannins, terpenoids (Moyo, Masika and
Muchenje, 2012; Kalpana, Moorthi and Kumara,
2013; Abdallah, 2016).
The results of identification by TLC method
against the ethanol fraction of M.oleifera leaf showed
that there was a class of polyphenol compounds
(Figure 5), alkaloids compounds (Figure 6),
flavonoids compounds (Figure 7), saponin
compounds (Figure 8), terpenoids compounds
(Figure 9). The results of identification of compounds
by TLC technique on spot color observation both
visually and irradiated by UV 254 nm and 365 nm,
obtained Rf value presented in Table 3.
Antibacterial and Characterization of Secondary Metabolite Compound from Ethyl Acetate and Ethanol Fraction of Leaves Moringa oliefera
L
235

Table 1: Antibacterial activity result of M.oleifera L. leaf fractionation against Staphylococcus aureus and Escherichia coli
Sample concentration Rf
Average diameter of inhibitory zone
Staphylococcus aureus Escherichia Coli
Ethyl Acetate Faction
50mg/mL
0.28 9.21±3.72 7.1±1.9
0.38 10.21±3.49 10.2±2.9
0.49 8.77 ±3.19 10.6±1.1
0.69 9.91 ±1.88 8.8±1.7
0.79 10.17±1.11 10.7±1.8
0.86 12.26±2.69 11.8±1.5
0.94 13.73±0.29 14.5±0.7
Ethanol Fraction
50mg/mL
0.18 5.46 ±2.53 10.13±2.68
0.33 8.34 ±3.05 9.39±2.08
0.38 9.70 ±3.22 9.30±1.30
0.75 7.67 ±5.20 10.06±2.54
0.92 14 ±2.53 10.86±2.91
Erythromycin 15µg/disk 38.7±1.41 -
Chloramphenicol 30µg/disk - 32.33±0.58
Figure 1: Identification of terpenoids compounds with TLC,
(a) UV 254 nm (b) UV 365 nm (c) derivatization, UV 365
nm (d) derivatization, visual.
Figure 2: Identification of flavonoids compounds with
TLC, (a) UV 254 nm (b) UV 365 nm (c) derivatization, UV
365 nm (d) derivatization, visual.
Figure 3: Identification of polyphenols compounds with
TLC (a) UV 254 nm (b) UV 365 nm (c) derivatization, UV
365 nm (d) derivatization, visual.
Figure 4: Identification of anthraquinone compounds with
TLC (a) UV 254 nm (b) UV 365 nm (c) derivatization, UV
365 nm (d) derivatization, visual.
HSIC 2019 - The Health Science International Conference
236
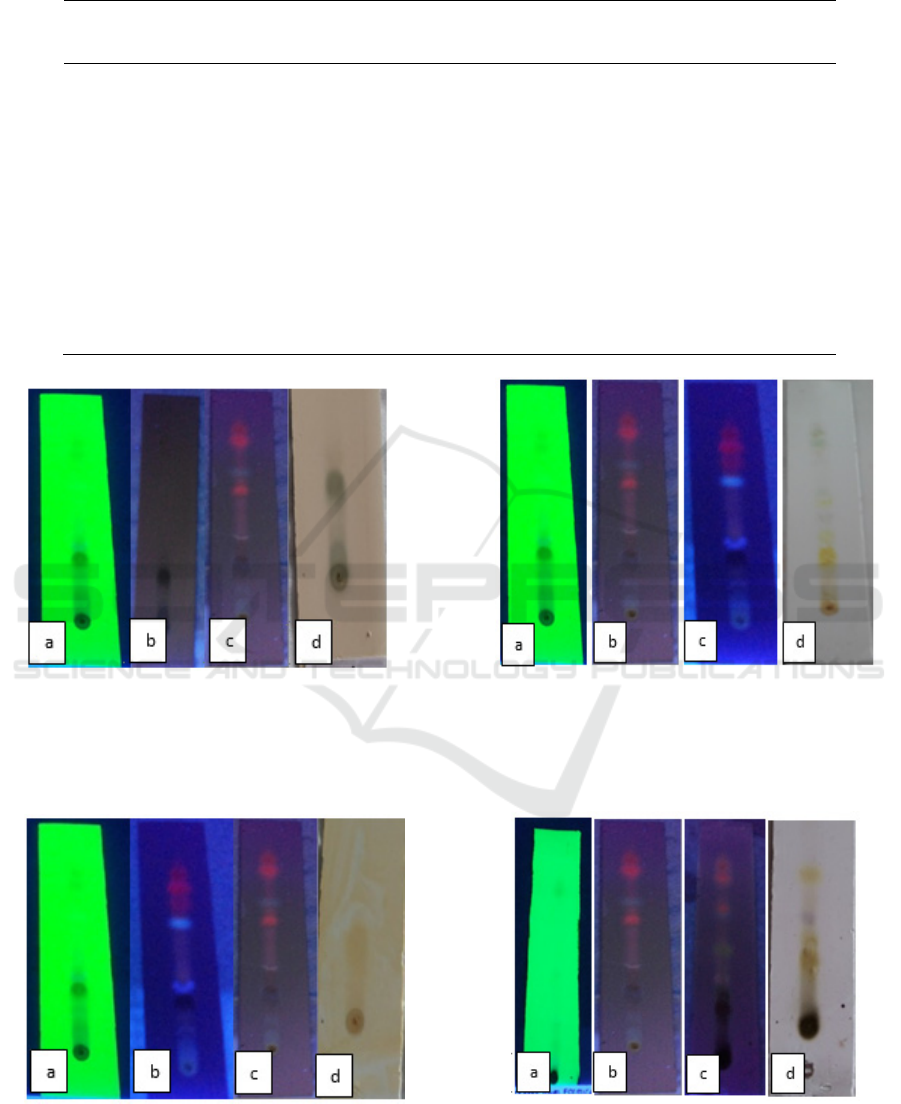
Table 2: TLC Results from the ethyl acetate fraction of M.oleifera leaves mobile phase N-Hexane : Ethyl Acetate (4: 6).
Rf
Flavonoids
(10% Sulfuric
Acid)
Terpenoid
(Anisaldehyde-Sulfuric
Acid)
Polyphenol
s ( FeCl
3
)
Anthraquinone
(KOH 10% in methanol)
0.2
8
- Purple red - -
0.3
8
- - - Red purple
0.4
9
- - - purple green
0.6
9
- Purple red -
0.7
9
Intensive yellow - - -
0.8
6
- Purple red - -
0.9
4
- - Black Brownish yellow
Figure 5: Identification of polyphenols compounds with
TLC (a) UV 254 nm (b) UV 365 nm (c) derivatization, UV
365 nm (d) derivatization, visual.
Figure 6: Identification of alkaloid compounds with TLC
(a) UV 254 nm (b) UV 365 nm (c) derivatization, UV 365
nm (d) derivatization, visual.
Figure 7: Identification of flavonoid compounds with TLC
(a) UV 254 nm (b) UV 365 nm (c) derivatization, UV 365
nm (d) derivatization, visual.
Figure 8: Identification of saponin compounds with TLC (a)
UV 254 nm (b) UV 365 nm (c) derivatization, UV 365 nm
(d) derivatization, visual.
Antibacterial and Characterization of Secondary Metabolite Compound from Ethyl Acetate and Ethanol Fraction of Leaves Moringa oliefera
L
237

Figure 9: Identification of terpenoid compounds with TLC (a) UV 254 nm (b) UV 365 nm (c) derivatization, UV 365 nm (d)
derivatization, visual.
Table 3. TLC results from the Ethole Acetate fraction of M.oleifera leaves mobile phase Ethyl acetate: n-Hexane: methanol
(4: 0.5: 0.5) added 1 drop of formic acid.
Rf
Polyphenol
s ( FeCl
3
)
Alkaloids
(Dragen-droff)
Saponin
(Anisaldehyde-
Sulfuric Acid)
Flavonoids
(10% Sulfuric
Acid)
Terpenoid
(Anisaldehyde-
Sulfuric Acid)
0,18 Black Orange - - -
0,33 - - - - -
0,38 - - purple - -
0,75 - - - Yellow Intensive -
0,92 - - - - purple
The secondary metabolite compounds detected in
the ethyl acetate and ethanol fraction have
antibacterial activity. The flavonoid antibacterial
mechanism works by inhibiting the synthesis of DNA
and RNA from bacteria. In Proteus vulgaris bacteria,
flavonoids show a process of inhibiting the formation
of strong bacterial DNA. Whereas in the process of
inhibiting the formation of bacterial RNA the
strongest results were found in S. aureus. In addition
to inhibiting flavonoid DNA and RNA synthesis it
also inhibits the formation of bacterial cytoplasmic
membranes. Examples of antibacterial flavonoids are
apigenin, quercetin, flavonone, isoflavones, luteolin,
and derivatives of epigalotekin (Patel et al., 2014).
The terpenoid compound as an antibacterial works
by inhibiting bacterial growth through destruction in
the bacterial cell membrane. In the polyphenol group,
antibacterial activity works by binding to proteins,
damaging cell membranes, and inhibiting the reverse
transcriptase enzyme so that bacterial cells cannot be
formed (Emmanuel et al., 2014).
In this study also found the antrakinon group. In
this group has a broad antibacterial activity.
Anthraconone works by forming complexes with
nucleophilic amino acids in proteins that can cause
proteins to lose their function. Quinone reacts with
cell hair adhesion proteins, cell wall polypeptides,
and echoenzymes released through membranes
(Putra, 2010).
Polyphenolic compounds can also have
antibacterial activity. The mechanism of action of
polyphenols as an antibacterial is by binding to
proteins, damaging bacterial cell membranes and
inhibiting enzyme expenditure. Inhibiting the reverse
transcriptase enzyme (the reverse transcription
process that is copying RNA into DNA) and DNA
topoisomerase (curling) so that bacterial cells cannot
be formed (Nuria, Faizatun and Sumantri, 2009).
Alkaloid compounds contain nitrogen groups and
are usually present in high amounts in certain plant
parts. This compound is usually found in seeds, fruit,
leaves, roots and on the bark. One of the functions of
alkaloids is as a poison to protect plants from animal
and insect attacks, but some are used as treatments
such as morphine and quinine. Alkaloid compounds
can interfere with the formation of cross bridges of
peptidoglycan compounds in bacterial cells so that the
cell wall layer is not formed intact which then results
in cell death (Patel et al., 2014).
The mechanism of action of saponin as an
antibacterial is that it can cause leakage of proteins
and enzymes from within the cell. Saponins can be
anti-bacterial because of their surface active
substances which reduce the surface tension of
bacterial cell walls and damage membrane
HSIC 2019 - The Health Science International Conference
238

permebiality. Damage to the cell membrane is very
disturbing survival of bacteria. Saponins diffuse
through the outer membrane and cell walls of the
vulnerable and then bind to the cytoplasmic
membrane so that it interferes with and reduces the
stability of the cell membrane (Kalpana, Moorthi and
Kumara, 2013).
4 CONCLUSIONS
The compounds in the ethyl acetate fraction show the
class of compounds Flavonoids, Terpenoids,
Polyphenols and Anthraquinone while in the ethanol
fraction Moringa oleifera L. leaves show the
compounds of the compounds Alkaloids, Flavonoids,
Terpenoids, Polyphenols, and Saponin while in the
ethanol fraction Moringa oleifera L. Antimicrobial
activity is shown in the ethyl acetate and ethanol
fraction in both Staphylococcus aureus and
Escherichia coli.
REFFERENCES
Abdallah, E. (2016) ‘Antibacterial properties of leaf extracts
of Moringa oleifera Lam. growing in Sudan’, Journal of
Advances in Medical and Pharmaceutical Sciences,
5(1), pp. 1–5. Available at:
https://pdfs.semanticscholar.org/39bc/e9f1d9288cca16c
1a0383ab5b62eb09d3e52.pdf (Accessed: 18 November
2019).
Akinyeye, A., Solanke, E. and Adebiyi, I. (2014)
‘Phytochemical and antimicrobial evaluation of leaf and
seed of Moringa olifera extracts’, Int. J. Res. In Med,
4(6). Available at:
https://pdfs.semanticscholar.org/f4ee/2b2d6aedc5f9ae8
65a89ea6d53a7b4ac1b3c.pdf (Accessed: 18 November
2019).
Brooks, G. F. et al. (2013) Medical Microbiology. 26th edn.
New York: McGraw-Hill Companies.
Emmanuel, S. et al. (2014) ‘Phytochemical and
antimicrobial studies of methanol, ethyl acetate, and
aqueous extracts of Moringa oleifera seeds’, American
Journal of Ethnomedicine, 1(5). Available at:
https://www.researchgate.net/profile/Olajide_Olutayo/p
ublication/332528284_Phytochemical_and_Antimicrob
ial_Studies_of_Methanol_Ethyl_acetate_and_Aqueous
_Extracts_of_Moringa_oleifera_Seeds/links/5ae04180a
ca272fdaf8bd9b4/Phytochemical-and-Antimicrobial-
Stud (Accessed: 19 November 2019).
Gyawali, R. and Ibrahim, S. A. (2014) ‘Natural products as
antimicrobial agents’, Food Control. Elsevier, 46, pp.
412–429. doi: 10.1016/J.FOODCONT.2014.05.047.
Kalpana, S., Moorthi, S. and Kumara, S. (2013)
‘Antimicrobial activity of different extracts of leaf of
Moringa oleifera (Lam) against gram positive and gram
negative bacteria’, International Journal of Current
Microbiology, 12(2).
Luqman, S. et al. (2012) ‘Experimental Assessment of
Moringa oleifera Leaf and Fruit for Its Antistress,
Antioxidant, and Scavenging Potential Using In Vitro
and In Vivo Assays.’, Evidence-based complementary
and alternative medicine : eCAM. Hindawi, 2012, p.
519084. doi: 10.1155/2012/519084.
Minaiyan, M. et al. (2014) ‘Anti-inflammatory effect of
Moringa oleifera Lam. seeds on acetic acid-induced
acute colitis in rats.’, Avicenna journal of
phytomedicine. Mashhad University of Medical
Sciences, 4(2), pp. 127–36. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25050310
(Accessed: 18 November 2019).
Moyo, B., Masika, P. and Muchenje, V. (2012)
‘Antimicrobial activities of Moringa oleifera Lam leaf
extracts’, AFRICAN JOURNAL OF
BIOTECHNOLOGY. Academic Journals (Kenya),
11(11), pp. 2797–2802. doi: 10.5897/AJB10.686.
Nuria, M., Faizatun, A. and Sumantri, S. (2009) ‘Uji
Aktivitas Antibakteri Ekstrak Etanol Daun Jarak Pagar
(Jatropha Curcas L) Terhadap Bakteri Staphylococcus
aureus ATCC 25923, Escherichia coli ATCC’,
Mediagro, 5(2). Available at:
https://www.publikasiilmiah.unwahas.ac.id/index.php/
Mediagro/article/download/559/680 (Accessed: 19
November 2019).
Oluduro, A. O. (2012) ‘Evaluation of Antimicrobial
properties and nutritional potentials of Moringa
oleiferaLam.leaf in South-Western Nigeria’, Malaysian
Journal of Microbiology, 8(2), pp. 59–67.
Patel, P. et al. (2014) ‘Phytochemical analysis and
antifungal activity of Moringa oleifera’, Journal of
Pharmacy, 6(5). Available at: https://pdfs.
semanticscholar.org/63c9/b2bd4b4dd79522ccfaeabf08
3bcb5a04be69.pdf (Accessed: 19 November 2019).
Putra, I. (2010) ‘AKTIVITAS ANTIBAKTERI EKSTRAK
KULIT BUAH MANGGIS (Garcinia mangostana L.)
SERTA KANDUNGAN SENYAWA AKTIFNYA
[Antibacterial Activity of’, Jurnal Teknologi Pangan
dan Industri, 21(1). Available at:
http://journal.ipb.ac.id/index.php/jtip/article/view/2422
(Accessed: 19 November 2019).
Sarker, S. D., Latif, Z. and Gray, A. I. (2006) ‘Natural
Product Isolation’, in Natural Products Isolation.
Totowa, NJ: Humana Press, pp. 1–25. doi: 10.1385/1-
59259-955-9:1.
Wikansari, (Nurvita) (2012) ‘Pemeriksaan Total Kuman
Udara Dan Staphylococcus Aureus Di Ruang Rawat
Inap Rumah Sakit X Kota Semarang’, Jurnal Kesehatan
Masyarakat Universitas Diponegoro. Diponegoro
University, 1(2), p. 18795. Available at:
https://www.neliti.com/publications/18795/pemeriksaa
n-total-kuman-udara-dan-staphylococcus-aureus-di-
ruang-rawat-inap-ruma (Accessed: 18 November 2019).
Yacob, T. et al. (2011) ‘Resistensi Antibakteri pada Pasien
Infeksi Saluran Kemih (ISK) dengan Kateterisasi Urin
di Bagian Penyakit Dalam RSUD Arifin Achmad
Pekanbaru’, Jurnal Ilmu Kedokteran, 5(2), pp. 94–100.
Antibacterial and Characterization of Secondary Metabolite Compound from Ethyl Acetate and Ethanol Fraction of Leaves Moringa oliefera
L
239
