
HPMC Inhibit Mannitol Re-crystallization in Air-dried Liposome
Formulations
Raditya Weka Nugraheni
1,2*,
Helmy Yusuf
2
, Dwi Setyawan
2
1
Department of Pharmacy, Faculty of Health Sciences, University of Muhammadiyah Malang Jalan Bendungan Sutami 188
A Malang 65145, East Java, Indonesia
2
Department of Pharmaceutics, Faculty of Pharmacy, Universitas Airlangga, Jalan Mulyorejo Surabaya 60115, East Java,
Indonesia
Keywords: Liposome, Air-Drying, Mannitol, HPMC, Crystallizations
Abstract: Liposome defined as a spherical vesicle, which is formed when phospholipids are being hydrated with an
aqueous environment. This study investigated the role of HPMC to inhibit mannitol crystallization in the air-
dried liposome formulation. HPMC has been used in solid dispersion to prevent crystallization drugs. The
mannitol and HPMC were included in the developed formulations with four different ratios and air-dried at
40ºC for 120 h. Liposome was composed of SPC:DDA: Chol (9:3:1 in molar ratio). XRD data showed
crystalline-forming properties as a function of mannitol and HPMC ratio. The formula with the highest
mannitol: HPMC ratio (4:1) showed the most apparent crystallinity, while the lowest proportion (2:3)
indicated amorphous solid. DTA analysis found that the following formula showed a broad endothermic peak
at 150-170ºC, indicating amorphous solid. SEM data supported these results where no crystalline structure
was observed in the latter formulation. The rest formula showed partially amorphous and partially crystalline.
It can be concluded that the incorporation of HPMC as a dispersion matrix potentially inhibits crystal
formation in the developed formulation
1 INTRODUCTION
Liposome was defined as a spherical vesicle, which is
formed when phospholipids are being hydrated with
an aqueous environment (Kaur, 2011). Liposome,
which is widely used in drug delivery, is well known
for its versatility for delivering both hydrophilic and
lipophilic agents(Çağdaş, Sezer, & Bucak, 2014;
Chen, Han, Cai, & Tang, 2010). Research on
producing liposomes into solid materials has been
conducted extensively since solid liposome has more
advantages in terms of stability compared to the
liposome in water suspension. Decreasing molecular
mobility in a solid-state may decrease chemical
degradation, which leads to physical instability
(Mohammed, Bramwell, Coombes, & Perrie, 2006).
However, dehydration could also be detrimental
for the liposome since removing water from the
system will cause the vesicle structure to collapse. To
prevent this, compounds that are rich in hydroxyl
group are employed to interact with the phosphate
head, thus replacing the water molecules during
drying (Franks, 2007; Ingvarsson, Yang, Nielsen,
Rantanen, & Foged, 2011). Mannitol is an alcoholic
sugar that is widely used in the drying process of the
liposome. However, mannitol is easily re-crystallized
and potentially damaging bi-layer the membrane (Li
et al., 2016).
We tried to address the problem by employing
hydroxypropylmethylcellulose
(HPMC) as a
dispersing matrix, which will help to suppress the re-
crystallization of mannitol (Kiew, Cheow, &
Hadinoto, 2015). HPMC is a cellulose-derived
polymer that methylated and hydroxy-propylated
(Rowe, 2009). It is widely used as bioadhesive
material, a controlled release agent, a dispersion
agent, and an increasing viscosity agent. To produce
a stable liposome vesicle, we used a combination of
soy phosphatidylcholine (SPC) as principal lipid
constituent, bromide salt of dimethyl
dioctadecyl
ammonium (DDAB) which is cationic, and
cholesterol (Patent No. US20150079156 A1, 2015).
We observed the effects of mannitol in
combination with HPMC as a dispersing matrix in
different ratios on the physical characteristics of the
solid dried products. Powder X-Ray Diffraction
228
Nugraheni, R., Yusuf, H. and Setyawan, D.
HPMC Inhibit Mannitol Re-crystallization in Air-dried Liposome Formulations.
DOI: 10.5220/0009130502280232
In Proceedings of the 2nd Health Science International Conference (HSIC 2019), pages 228-232
ISBN: 978-989-758-462-6
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(PXRD), and Differential Scanning Calorimetry
(DSC) were used to investigate the properties of the
products. The procedures of physical characterization
were conducted according to our previous work
(Yusuf, 2013).
2 METHODS
Materials
The lipid phase for liposome formulation was
Dimethyl-Dioctadecylammonium (Sigma Aldrich,
Singapore) and Soy Phosphatidylcholine (Lipoid
GmBh, Germany), and cholesterol (Sigma-Aldrich,
Singapore). The use of cholesterol was to enhance
liposomal membrane stability. The protectant used in
this research was Mannitol (Sigma-Aldrich,
Singapore), a poly-alcohol compound. Hydroxy-
propyl-methyl-cellulose (Shin-Etsu, Japan) was
selected as a dispersion matrix to increase physical
stability of the products in terms of increasing the
total mass of the products. Methanol (E. Merck) was
chosen to dissolve all of the lipid phases in the
liposomal ingredients. All materials used were of
analytical grade.
Research Procedure
The technique for liposome formula preparation was
thin-film hydration methods in which the lipid phase
solution was evaporated and hydrated using water
medium. The lipid phase was dissolved in methanol
proportionally SPC:DDA: Cholesterol = 9:3:1.
The thin-film, which was formed after the
evaporation using a rotary evaporator (Büchi,
Germany) for 60 minutes hydrated with a solution of
mannitol in various concentrations that had
previously warmed to facilitate miscibility. Had been
done at 50°C for 10 minutes, the appearance of white
liquid suspension indicate the formation of liposomal
suspension. Liposome suspension sonicated for 5
minutes to produce a smaller vesicle size. HPMC
powder was weighed
according to Table 1, and
dispersed in 5 mL purified water to form HPMC gel.
Liposome suspension was combined into HPMC
gel and stirred until a homogeneous mixture achieved
and portioned into vials
for air-drying. The
temperature and time of the air-drying was 40°C for
72 hours.
Table 1: The composition of Mannitol and HPMC in the
Formula
Formula
Mannitol
Weight (g)
HPMC
Weight (g)
Mannitol:
HPMC
FMO1 0,250 0,125 2:1
FMO2 0,500 0,125 4:1
FMO3 0,250 0,375 2:3
FMO4 0,500 0,375 3:4
Differential Thermal Analysis (DTA)
The thermal profiles of the solid samples were
analyzed using DTA instrument (Mettler Toledo,
Switzerland). The sample is put into aluminum
crucibles and scanned from 30°-300°C at a heating
rate of 10°C/min.
X-Ray Diffraction Analysis (XRD)
The crystallinity of the dried products analyzed using
Powder X-Ray Diffraction Instrument (Phillips
X'Pert PRO PANalytical, Netherlands). The samples
inserted into the sample holder and flatten. The
condition of analysis waa using Cu metal target and
Kα filter. The voltage and current were 40 kV and 40
mA, performed at room temperature, in a range of 2Ɵ
of 5-40°.
Scanning Electron Microscopy (SEM)
A small amount of the samples was dispersed and
glued onto 25 mm diameter sample holder. The
samples were sputter-coated with 5 nm layer of Au-
Pd (Gold-Palladium). The observation was completed
using 500 and 1000 magnitude.
3 RESULTS AND DISCUSSION
The formulations profile was partially amorphous.
The results could be seen from the presence of broad
peaks in the temperature range 96-116 for FMO1,
FMO2, and FMO3, which correspond to endothermic
transition temperatures for HPMC. The results show
that some of the material has been incorporated to
form amorphous solid dispersions and is the expected
profile of the product because it indicated the success
of the vitrification mechanism in maintaining the
physical stability of the dry liposome (Ingvarsson et
al., 2011).
HPMC Inhibit Mannitol Re-crystallization in Air-dried Liposome Formulations
229
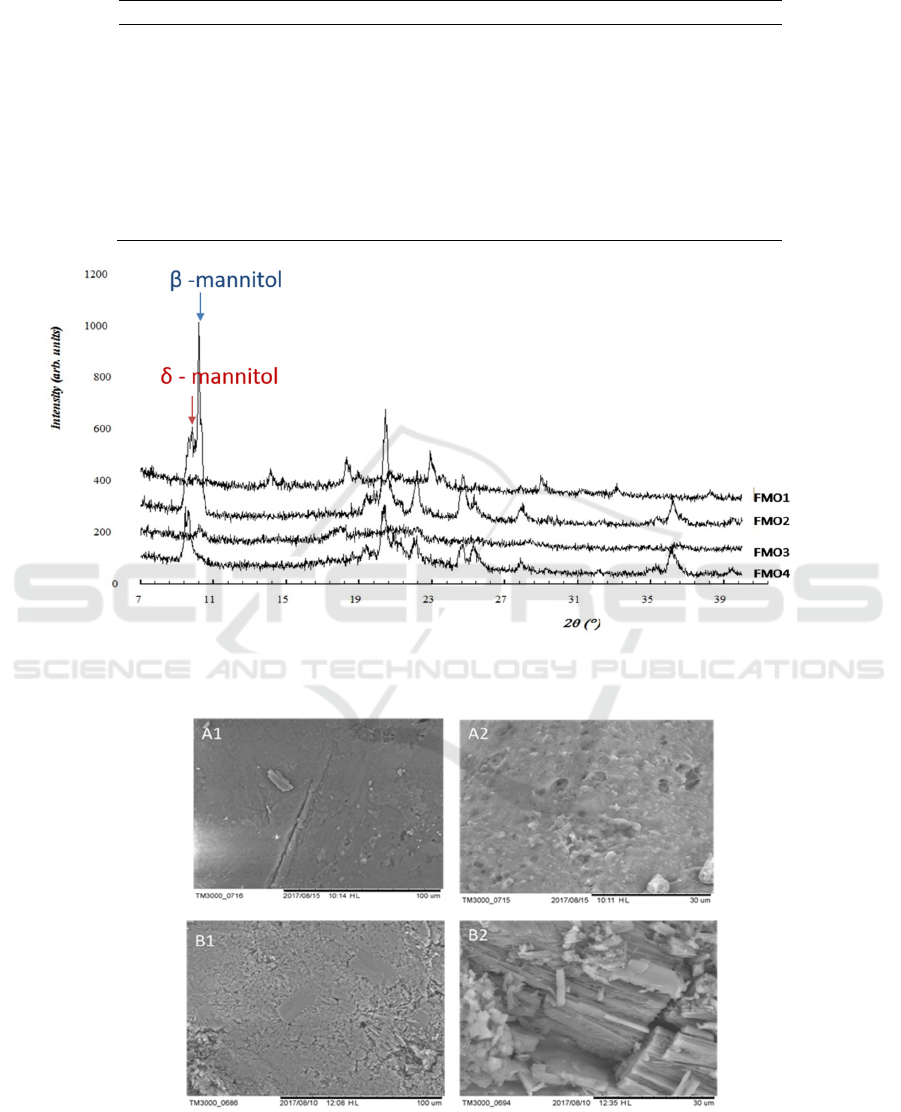
Table 2: Endothermic peak list in DTA thermogram
T1 (˚C) ΔH1 (J/g) T2 (˚C) ΔH2 (J/g) T3 (˚C) ΔH3 (J/g)
SPC 99.54 284.49
DDAB 90.18 145.50
Cholesterol 149.31 79.8
Mannitol 168.3 190
HPMC 88.37 169.95
FMO1 116.3 17.4 166.5 72.2
FMO2 96.7 10.7 157.4 9.50 172.5 113.0
FMO3 100.3 32.1 157.5 11.4 171.8 48.7
FMO4 160.3 62.3
Figure 2: The X-ray diffractogram profile of air-dried liposome formulation with mannitol in combination with HPMC in a
different ratio, according to table 1.
Figure 3: Morphology of liposome formulation characterized by SEM. A1 and A2 were FMO3, while B1 and B2 were FMO4.
HSIC 2019 - The Health Science International Conference
230

However, there are still sharp peaks that may
correlate with mannitol transition temperatures. The
results of the thermal analysis showed that there were
new peaks (listed in Table 2) different from the
original substances. Mannitol is a material which has
different morphological phases (polymorphism), β-
and δ-mannitol polymorph had endothermic peaks in
157˚ and 167˚ Celsius, respectively (Barreneche, Gil,
Sheth, Inés Fernández, & Cabeza, 2013). The peak of
pure mannitol was observed in 168.3˚C, indicating δ-
mannitol existence; no other peak was observed.
However, observations of thermal analysis of the
formulations showed two peaks that existed together
in FMO2 and FMO3. From this data, we could
conclude that β-mannitol was formed during the
formulations processes as there was no raw material
which had endothermic peaks at such temperature.
Interestingly, only β-mannitol observed in FMO1,
and only δ-mannitol was found in FMO4. The
difference between the FMO1 and FMO4 formulas is
in the ratio of the weight of mannitol: HPMC, which
are 2:1 and 3:4, respectively. Mannitol levels in
FMO1 are higher than HPMC, so the shape of the
delta mannitol, which has existed since the beginning,
is relatively unchanged. Whereas in FMO3, the
HPMC ratio is higher than that of mannitol; this is
what might trigger the shift from delta to beta
mannitol. High HPMC ratios also occur in FMO3, but
in this formula, the amorphous form dominates, as
evidenced by the relatively high endothermic
enthalpy at 100.3 (ΔH1=32,1 J/g). The differences in
endothermic enthalpies of the peaks indicating
different energy levels of thermodynamic transitions
in the formulations. These results will be confirmed
using the crystallinity profile from X-Ray powder
diffraction.
The X-Ray diffractogram results showed that
FMO3 was an amorphous form, characterized by the
absence of intensive crystalline peaks (Kiew et al.,
2015). Nevertheless, the sharp peak of mannitol
detected from the formulas FMO2 and FMO4.
According to the previous investigations, the
characteristic peaks of β-mannitol was 10.56° and
14.71°, the α-mannitol was 13.79°, and δ-mannitol
was 9.57° (Vanhoorne et al., 2016). In FMO2, it is
confirmed that there is a mixture of β- and δ-mannitol
all at once. The results show that the difference in the
ratio of mannitol: HPMC in the formula is very
influential on the phase behavior even though
although the process is carried out uniformly.
Morphological analysis with SEM instruments
also supports the DTA and XRD examination. The
FMO3 formula is known to provide an amorphous
and porous surface image, while FMO4 on the same
scale shows a high crystallinity (Haque & Roos,
2005). As a carrier for dry liposomes, the FMO3
profile is preferrable because liposomes can be
incorporated and protected during the drying process
(Nugraheni, Setyawan, & Yusuf, 2017).
4 CONCLUSIONS
The incorporation of HPMC as a dispersion matrix
potentially inhibits crystal formation in the developed
formulation, especially in FMO3, which is the most
suitable carrier for air-dried liposome compared to the
other formulations.
REFERENCES
Barreneche, C., Gil, A., Sheth, F., Inés Fernández, A., &
Cabeza, L. F. (2013). Effect of d-mannitol
polymorphism in its thermal energy storage capacity
when it is used as PCM. Solar Energy, 94, 344–351.
https://doi.org/10.1016/j.solener.2013.05.023
Çağdaş, M., Sezer, A. D., & Bucak, S. (2014). Liposomes
as Potential Drug Carrier Systems for Drug Delivery. In
A. D. Sezer (Ed.), Application of Nanotechnology in
Drug Delivery. Retrieved from
http://www.intechopen.com/books/application-of-
nanotechnology-in-drug-delivery/liposomes-as-
potential-drug-carrier-systems-for-drug-delivery
Chen, C., Han, D., Cai, C., & Tang, X. (2010). An overview
of liposome lyophilization and its future potential.
Journal of Controlled Release, 142(3), 299–311.
https://doi.org/10.1016/j.jconrel.2009.10.024
Franks, F. (2007). Freeze-drying of pharmaceuticals and
biopharmaceuticals: Principles and practice.
Cambridge: Royal Society of Chemistry.
Haque, Md. K. & Roos, Y. H. (2005). Crystallization and
X-ray diffraction of spray-dried and freeze-dried
amorphous lactose. Carbohydrate Research, 340(2),
293–301. https://doi.org/10.1016/j.carres.2004.11.026
Ingvarsson, P. T., Yang, M., Nielsen, H. M., Rantanen, J.,
& Foged, C. (2011). Stabilization of liposomes during
drying. Expert Opinion on Drug Delivery, 8(3), 375–
388. https://doi.org/10.1517/17425247.2011.553219
Kaur, R. (2011). Liposomes: Formulation and
characterisation as contrast agents and as vaccine
delivery systems (Ph.D., Aston University). Retrieved
from http://eprints.aston.ac.uk/15820/
Kett, V., Yusuf, H., McCarthy, H., & Chen, K. H. (2015).
Patent No. US20150079156 A1. Retrieved from
http://www.google.ch/patents/US20150079156
Kiew, T. Y., Cheow, W. S., & Hadinoto, K. (2015).
Preserving the supersaturation generation capability of
amorphous drug-polysaccharide nanoparticle complex
after freeze drying. International Journal of
HPMC Inhibit Mannitol Re-crystallization in Air-dried Liposome Formulations
231

Pharmaceutics, 484(1–2), 115–123.
https://doi.org/10.1016/j.ijpharm.2015.02.057
Li, J., Hu, M., Xu, H., Yu, X., Ye, F., Wang, K., … Zhang,
D. (2016). Influence of type and proportion of
lyoprotectants on lyophilized ginsenoside Rg3
liposomes. Journal of Pharmacy and Pharmacology,
68(1), 1–13. https://doi.org/10.1111/jphp.12489
Mohammed, A. R., Bramwell, V. W., Coombes, A. G. A.,
& Perrie, Y. (2006). Lyophilisation and sterilisation of
liposomal vaccines to produce stable and sterile
products. Methods, 40(1), 30–38.
https://doi.org/10.1016/j.ymeth.2006.05.025
Nugraheni, R. W., Setyawan, D., & Yusuf, H. (2017).
Physical Characteristics of Liposomal Formulation
Dispersed in HPMC Matrix and Freeze-Dried Using
Maltodextrin and Mannitol as Lyoprotectant.
Pharmaceutical Sciences, 23(4), 285–292.
https://doi.org/10.15171/PS.2017.42
Rowe, R. C. (Ed.). (2009). Handbook of pharmaceutical
excipients (6. ed). London: APhA, (PhP)
Pharmaceutical Press.
Vanhoorne, V., Van Bockstal, P.-J., Van Snick, B., Peeters,
E., Monteyne, T., Gomes, P., … Vervaet, C. (2016).
Continuous manufacturing of delta mannitol by cospray
drying with PVP. International Journal of
Pharmaceutics, 501(1–2), 139–147.
https://doi.org/10.1016/j.ijpharm.2016.02.001
Yusuf, H. (2013). THE DEVELOPMENT OF FREEZE-
DRIED LIPOSOME FORMULATIONS AS VACCINE
DELIVERY SYSTEMS (Ph. D. Thesis). Queen’s
University Belfast, Belfast.
HSIC 2019 - The Health Science International Conference
232
