
The Effectiveness of Rice Husk Biochar Application to Metsulfuron
Methyl Persistence
Subhan Arridho, Saripah Ulpah and Tengku Edy Sabli
Department of Agrotechnology, Universitas Islam Riau, Pekanbaru, Indonesia
Keywords:
Herbicide, Metsulfuron Methyl, Persistence, Leaching, Rice Husk Biochar.
Abstract:
Metsulfuron methyl is an herbicide which has low toxicity and rapidly degraded in the soil, however DuPont
stated that it is very poisonous to aquatic organism. Rice husk biochar is commonly used as ameliorants,
moreoverit has ability to absorb and degradeharmful chemicals. This study aimed at investigating the
effectiveness of rice husk biochar application towards the persistence of metsulfuronmethyl in soil and seepage
water. This study applied completely randomized design factorial with two levels of herbicide dose (0 and 300
gr/ha) and four levels of percentage of rice husk biochar(0%, 5%, 10% and 15% of total soil). The results of
this research revealed that there was no metsulfuron methyl residue in soil of all treatments after 28 days of
herbicide treatment. The residue was found in seepageas much as 7.7 µg/L in treatment of 0% husk biochar
and 6.8 µg/L in treatment of 5%husk biochar. The seepage reduced by the increasing of the percentage of rice
husk biochar application. Thus, it can be concluded that giving the rice husk biochar is effective for absorbing
metsulfuron methyl and preventing it from leaching. However, itcould not hold the presence of metsulfuron
methyl longer in soil.
1 INTRODUCTION
Metsulfuron methyl is an herbicide active substance
which has low toxicity (LD50 in mice > 5000 mg/
kg), a low recommended dosage, and is also rapidly
degraded in the soil. Devlin et al. (1992) reported
thatmetsulfuron methyl, known as Ally, contains
DT50 for 2-4 weeks. However, in the DuPont Safety
Data Sheet, it is explained that Ally 20 WG(20%
metsulfuron methyl) is very poisonous to aquatic
organisms; it can cause long-term adverse effects in
the aquatic environment.
Persistence is the ability of the herbicide to
remain on the ground in an active state. The longer
the persistence of herbicides in the soil, the more
beneficial it will be, in terms of efficacy. However,
from an ecological perspective which is related
to environmental quality, the too-long persistence
of herbicides is certainly undesirable and should
be avoided because it will pollute the surrounding
environment. The persistence of herbicides in
the soil is influenced by several factors including:
volatilization, photodecomposition, adsorption,
leaching, microbial decomposition, chemical
decomposition, and uptake by plants (Rao, 2000).
Meanwhile, Jansar and Sahid (2016) stated that the
level of metsulfuronmethyl residue in the river near
oil palm plantations significantly increased during
the rainy season because of leaching.
Rice husk biochar is commonly used as
ameliorants in agricultural cultivation to improve
soil quality by improving the physical, chemical and
biological properties of the soil. In addition, rice
husk biochar is also known to have the ability to
absorb agricultural chemicals and it is decomposed
in physically, chemically and biologically into
compounds that are not harmful for the environment.
Jing et al. (2018) assert that giving rice husk
biochar could reduce the loss of ethyl phenoxaprop
herbicides in the soil, and decreased the toxic effects
to earthworms. Moreover, Sudirja et al. (2015) stated
that the adsorption of paraquat herbicides by soil
increases in line with the increasing doses of zeolite,
straw, and activated charcoal in the soil.
This research was conducted to investigate the
effectiveness of giving rice husk biochar ameliorant
towards the persistence of metsulfuron methyl in soil
and seepage water.
80
Arridho, S., Ulpah, S. and Sabli, T.
The Effectiveness of Rice Husk Biochar Application to Metsulfuron Methyl Persistence.
DOI: 10.5220/0009119600800084
In Proceedings of the Second International Conference on Science, Engineering and Technology (ICoSET 2019), pages 80-84
ISBN: 978-989-758-463-3
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIAL AND METHODS
2.1 Materials
The The materials applied in this research include:
Ally 20 WG herbicide, top soil, rice husk biochar,
98% purified metsulfuron methyl solution (brand:
Sigma Aldrich), methanol gred HPLC, acetonitrile,
acetic acid, KH
2
PO
4
, NaHCO
3
, HCl, and distilled
water.
The tools include: plastic pots,
Krisbow-semiautomatic handsprayers, scales,
Agilent 1220 Infinity LC HPLC-VWD,
OpenlabChemstation Software, Gyrozen
centrifugation machines, hot plate stirrers
Thermolyne, orbital shaker Protech, Sartorius
analytic scales, Sartorius pH meters, ultrasonic
machines, Biotage ISL Isolate Env+ Solid Phase
Extraction (SPE) cartridge, manifold vacuum,
vacuum pump, syringe, 0.2 micron syringe filter,
analysis vial bottle, beaker glass, volumetric flask,
centrifuge tube, flask, measuring tube, glass bottle 20
ml, and micropipette.
2.2 Research Site and Methodology
This research was conducted in the green house of
the experimental garden at the Faculty of Agriculture,
Universitas Islam Riau. The extraction and residual
analysis of the active substance of metsulfuron methyl
herbicide was executed in the pesticide analysis
laboratory of the Faculty of Science and Technology,
UniversitiKebangsaan Malaysia. The research was
conducted from April to June 2018. This research
applied a completely randomized design (CRD)
factorial pattern with two levels of herbicide dose (0
and 300 gr/ha) and four levels of percentage of rice
husk biochar ameliorant (0%, 5%, 10% and 15% of
total soil). The total amount of soil and rice husk
biochar in the pot is 2 kg, which was mixed evenly
(Figure 1). The treatment was repeated 3 times, so
that there were 24 units of the total experimental
treatments.
Herbicide was sprayed onto the ground with a
concentration of herbicide application 0.67 gr/L of
water and a spray volume of 450 l/ha. Each pot
was watered with 200 ml of water after 17 days of
herbicide application daily. The water that seeped out
under the pot was collected to be analyzed.
2.3 Sampling
The soil with the same treatment was composited,
stirred evenly, aerated for 2 hours then taken as much
Figure 1: Mixture of soil and rice husk biochar.
as 500 grams per treatment. Meanwhile, seeped out
water from pots that have been collected each day
was taken as the sample for as much as ± 250 ml per
treatment and it was put in a glass bottle.
2.4 Metsulfuron Methyl Extraction
For the extraction of metsulfuron methyl in the soil,
5 grams of soil sample were prepared, then mixed
with 0.1 M NaHCO
3
. The samples were shaken with
orbital shaker (200 rpm, 2 hours). After that, they
were centrifuged for 20 minutes at 4000 rpm. The
SPE cartridge was rinsed with 3 ml of acetonitrile
and 3 ml of distilled water. The supernatant resulted
was flowed about 2-3 ml per minute through the
SPE cartridge (Figure 2). Then, metsulfuron methyl
absorbed in the SPE cartridge was separated with
methanol and stored in a 20 ml glass bottle. The
extraction results were dried to a range of 1 ml. After
that, it was sucked with a syringe equipped with a 0.2
micron filter, then transferred to a 1.5 ml analysis vial
bottle.
Figure 2: Soil supernatant was flowed through SPE
cartridge for absorbingmetsulfuron methyl.
For the extraction of metsulfuron methyl in
seepage, 250 ml of seepage water samples were
prepared in a glass bottle. The pH of the water sample
was adjusted between 5-6 with potassium hydroxide
and or hydrochloric acid. The cartridge was rinsed
The Effectiveness of Rice Husk Biochar Application to Metsulfuron Methyl Persistence
81
with 3 ml of acetonitrile and 3 ml of distilled water.
Then, the water sample was flowed about 2-3 ml
per minute through the SPE cartridge. Next, the
metsulfuron methyl that was absorbed in the SPE
cartridge was separated with methanol and stored in
a 20 ml glass bottle. The extraction results were
dried to a range of 1 ml. After that, it was sucked
with a syringe equipped with a 0.2 micron filter, then
transferred to a 1.5 ml analysis vial bottle.
2.5 Metsulfuron Methyl Analysis
To provide a standard metsulfuron methyl primary
solution, 1.02 mg metsulfuron methyl (98% purity)
was weighed and dissolved with 50 ml of methanol
gred HPLC to produce a solution with a concentration
of 20 mg/L. Then, it was diluted so that the
concentration became 10 mg/L.
Next, to produce a standard metsulfuron methyl
curve, 5 series of secondary solutions were
formulated with concentrations of 50 µg/L, 100
µg/L, 200 µg/L, 300 µg/L and 500 µg/L respectively.
A secondary solution was formulated by dissolving
the primary solution as much as 0.05 ml, 0.1 ml,
0.2 ml, 0.3 ml, and 0.5 ml with methanol gred
HPLC until the solution volume became 10 ml in a
volumetric flask. All the solutions made were placed
in an ultrasonic device for 20 minutes and then
injected into a 1.5 ml vial analysis bottle by filtering
it using 0.2 micron filters to be analyzed using HPLC.
Furthermore, the standard solution of metsulfuron
methyl, methanol, soil samples and water samples
stored in 1.5 ml vial bottle was inserted into the
Agilent 1220 Infinity LC HPLC. Samples of each vial
bottle were automatically analyzed for 18 minutes and
the results of the analysis were displayed through the
OpenlabChemstation interface on a computer screen.
3 RESULTS AND DISCUSSION
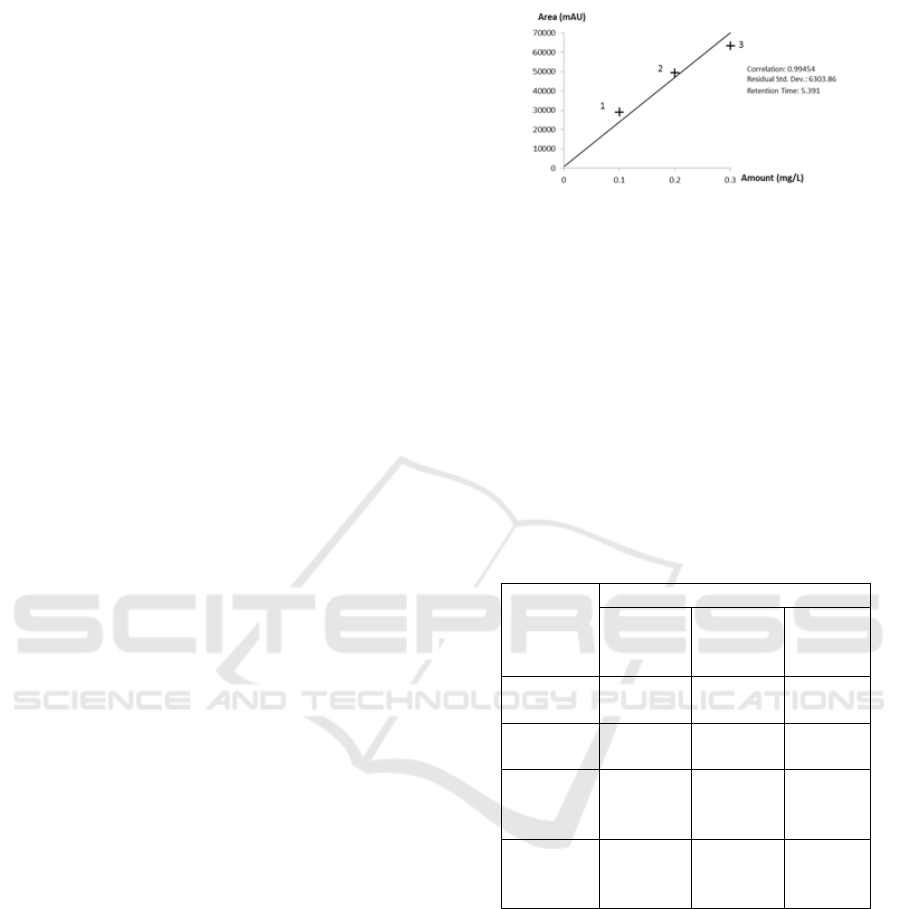
3.1 Calibration Curve
The highest correlation was obtained from a
combination of 3 series of metsulfuronmethyl
standard solutions, namely 100 g/L, 200 µg/L, and
300 µg/L, which had a correlation coefficient of
0.995. This implies that the concentration of standard
solutions gives an effect of 99% on the response
of the instrument, while the rest is influenced by
other variables.The above curve also shows that
metsulfuron methyl can be detected in the range of
RT (retention time) 5,391 minutes (Figure 3).
Figure 3: Linear regression curve of standard metsulfuron
methyl solution.
3.2 The Persistence of Metsulfuron
Methyl in Soil
Table 1 demonstrates that the metsulfuron methyl
residue was not found on the soil during HPLC
analysis. The ameliorant treatment of rice husk
biocharrevealed the same effect as the one without the
treatment of husk biochar after 28 days of herbicide
application. In other words, this research found that
rice husk biochar could not maintain the persistence
of metsulfuron methyl longer in the soil.
Table 1: Level of metsulfuron methyl residues in soiln
Treatment
Metsulfuron Methyl
Ret
Time
(minute)
Area
(mAU*s)
Residual
Level
(µg/L)
0% husk
biochar
5.391 0 0
5% husk
biochar
5.391 0 0
10%
husk
biochar
5.391 0 0
15%
husk
biochar
5.391 0 0
One of the important processes that control the
behavior of herbicides in the soil is the adsorption
carried out by the soil components. Herbicides can
be found in soil in the form of dissolved molecules
of the liquid phase and/or molecules that are bound
to soil phases such as minerals, organic matter, plant
residues, etc. (Zanini et al., 2008). In addition,
more than 36.3% to 55.7% of the applied metsulfuron
methyl turns into a residual form that binds to the soil
(Pons and Baniuso, 1998; Xu et al., 2002; Wang et
al., 2002). However, how the mechanism of colloidal
soil holds metsulfuron methyl and its metabolites is
still not clearly confirmed. Possible bonds between
herbicides and colloidal soils include: (1) ionic bonds,
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
82

(2) hydrogen bonds, (3) van der waals forces, (4)
ligand exchanges, (5) charge transfer complexes, (6)
hydrophobic partitioning, (7) covalent bonds and (8)
sequestration (Gevao et al., 2000).
Based on the results of this research, the
degradation of metsulfuron methyl herbicide in the
soil is resulted from through several degradation
processes, namely: hydrolysis, photolysis and
microbial decomposition. However, the authors
assume that the degradation of metsulfuron methyl
was more influenced by chemical degradation
(hydrolysis) than by biochemistry (microorganisms)
or physics (photolysis). This is in accordance with
Devlin et. al. (1992) who reported that the
degradation of sulfonylurea herbicides, such as Ally
Classic and Glean, is mostly caused by hydrolysis.
It is supported by a research conducted by Manna
(2015), which reported that the main mechanism of
chemical degradation of sulfonylurea herbicides is
caused by hydrolysis.
3.3 The Levels of Methyl Metsulfuron
Residue in Seepage
Based on the results of HPLC analysis, it was found
that the residual level of metsulfuron methyl in
seepage was 7.7 µg/L in the treatment of 0% husk
biochar and 6.8 µg/L in the treatment of 5% husk
biochar. Whereas, there was no metsulfuron methyl
residue was found in seepage at 10% husk biochar
and 15% husk biochar(table 2). This shows that the
level of residual metsulfuron methyl in seepage water
tends to decrease with the increasing amount of rice
husk biochar applied.
Table 2: Level of metsulfuron methyl residues in seepage
water
Treatment
Metsulfuron Methyl
Ret
Time
(minute)
Area
(mAU*s)
Residual
Level
(µg/L)
0% husk
biochar
5.365 1745.06 7.68
5% husk
biochar
5.357 1533.71 6.75
10%
husk
biochar
5.391 0 0
15%
husk
biochar
5.391 0 0
The authors believe that this tendency occured
due to the adsorption by rice husk biochar applied
in the soil, preventingmetsulfuron methyl from being
leached. Hence, it can be concluded that the addition
of rice husk biochar in this research is very effective
to prevent metsulfuron methyl from leaching. As a
result, it can reduce the negative impacts that arise in
the water ecosystem around it.
This research finding is in accordance with that
of by Zhelezova et al. (2017) who reported that
adding wood charcoal to sandy and clay soils cause
the adsorption of diuron herbicides increased. The
increasing of diuron adsorption in line with the
addition of charcoal, because charcoal has many
absorbent surfaces that can bind non-polar herbicides
so it can reduce the risk of leaching. Jing et al. (2018)
investigated that the addition of rice husk biochar
could slow the loss of ethyl phenoxaprop herbicide
in the soil.
The prevention of metsulfuron methyl leaching
can certainly be used as a solution to prevent
the contamination of active substance of herbicides
reaching to underground water and other water
ecosystems such as rivers and lakes.
A very low residual level of metsulfuron
methyl does not mean have no negative impact
on the environment. Fairchild (1995) reported
that metsulfuron methyl could cause the reduction
of 50% of the number of Lemna minor leaves
in a period of 14 days with an EC50 0.4
µg/L. If it accumulates continuously over a long
period of time, it is not impossible that other
aquatic organisms can be affected, including:
algae (Selenastrumcapricornutum, EbC
50
3.9 mg/L),
crustaceans (Daphnia magna, EC
50
> 150 mg/L), and
fish (Bluegill sunfish, LC
50
> 150 mg/L).
4 CONCLUSIONS
Metsulfuron methyl was completely degraded 28 days
after herbicide application regardless the application
of rice huskbiochar, which is assumed to be caused
by hydrolysis as the main factor.
The residual metsulfuron methyl was found in
seepage water in the treatment of 0% husk biocharas
much as 7.7 µg/L and in the treatment of 5% husk
biochar as much as 6.8 µg/L, while the treatment of
10% and 15% husk biochar was 0 µg/L. This indicates
that the addition of rice husk charcoal ameliorant
is very effective in absorbing and breaking down
metsulfuron methyl in the soil, so that the further
contamination of herbicide metsulfuron methyl into
the surrounding water environment can be avoided.
The Effectiveness of Rice Husk Biochar Application to Metsulfuron Methyl Persistence
83

ACKNOWLEDGMENTS
The authors wish to thank Universitas Islam Riau
for funding this publication and Centre for Earth
Sciences and Environment, Faculty of Science and
Technology, Universiti Kebangsaan Malaysia for
providing technical guidance and research fasilities.
REFERENCES
Devlin, D. L., Peterson, D. E., and Regehr, D. L. 1992.
Residual Herbicides, Degradation and Recropping
Interval.
Fairchild, J. F., Ruessier, D. S., Lovely, P. A., Whites,
D. A., and Heine, P. R. 1995. An Aquatic
Plant Risk Assessment of Sixteen Herbicides Using
Toxicity Tests with Selenastrumcapricornutum and
Lemna minor.
Gevao, B., Semple, K. T., and Jones, K. C. 2000. Bound
Pesticide Residue in Soils: A review. Environmental
Pollution, 108:3–14.
Jansar, K. M. and Sahid, I. B. (20). 2016. Residue
Determination and Monitoring of The Levels of
Metsulfuron Methyl in Selected Rivers at TasikChini
Pahang Malaysia. Malaysian Journal of Analytical
Sciences 20.
Jing, X., Wang, T., Yang, J., Wang, Y., and Xu, H.
2018. Effect of Biochar on The Fate and Toxicity of
Herbicide Fenoxaprop-Ethyl in Soil. R. Soc. Open Sci,
5:171875.
Pons, N. and Baniuso, E. 1998. Fate of metsulfuron-methyl
in soils in relation to pedo-climatic conditions. Pestic.
Sci, 53:311–323.
PT. (20). DuPont Agricultural Products Indonesia. Lembar
Data Keselamatan Ally 20 WDG.
Rao, V. S. (2000). Principle of Weed Science 2nd Eds.
Science Publisher, Inc.
S., M. (2015). Effect of Biochar Amendments on Fate of
Pyrazosulfuron-Ethyl in Soil.
Sudirja, R., Arifin, M., and Joy, B. 2015.
AdsorpsiParaquatdanSifat Tanah padaTigaSubgrup
Tanah AkibatPemberianAmelioran.
JurnalAgrikultura, 26.
Wang, H. Z., Xu, J. M., Xie, Z. M., and Ye, Q. F. 2002.
Dynamics of bound residues of metsulfuron-methyl in
soil humus. Acta Sci. Curcum. 22, 22.
Xu, J. M., Wang, H. Z., Xie, Z. M., and Chen, Z. L. 2002.
Distribution of bound residues of metsulfuron-methyl
in soil combined humus. China Environ. Sci. 22, 22.
Zanini, G. P., Maneiro, C., Waiman, C., Galantini, J. A., and
Rosell, R. A. 2009. Adsorption of Metsulfuron Methyl
on Soils under No-Till System in Semiarid Pampean
Region, Argentina. Geoderma, 149:110–115.
Zhelezova, A., Cederlund, H., and Stenstrom, J. 2017.
Effect of Biochar Amendment and Ageing on
Adsorption and Degradation of Two Herbicides.
Water Air Soil Pollut, 228:216.
ICoSET 2019 - The Second International Conference on Science, Engineering and Technology
84
