
Application of Mirror Neuron System in Post Stroke Rehabilitation
Vitriana Biben, Jihan Alifa Syahida
Department of Physical Medicine and Rehabilitation, Dr. Hasan Sadikin General Hospital,
Faculty of Medicine, University of Padjadjaran, Bandung, Indonesia
vitriana@unpad.ac.id
Keywords: Mirror Neuron System, Motor Imagery, Virtual Reality
Abstract: Skill development by enhancing experience-dependent plasticity using mirror neuron system through
motor imagery and or virtual reality approach has been increasing nowadays. Mirror neuron as a
visuomotor neuron will activated in relation to movement of body parts or in observation of the actions.
Several studies have examined this network and properties in humans and prove the mechanisms in
enhancing neuroplasticity. Although there are many studies for on the mirror neuron system, several
questions remain unanswered. The motor imagery and virtual reality as the practical approach studies in
post stroke rehabilitation also showed none of this approach was absolutely superior to each other. To
increase the comprehension of mirror neuron system involvement in post stroke rehabilitation, this article
is try to focusing on the review of motor imagery and virtual reality approach that use the principle.
1 INTRODUCTION
Stroke is the most common acquired neurological
disease in the adult population and a leading cause
of disabilities worldwide (Aqueveque et al.,
2017),(García-Rudolph et al., 2019). The prevalence
of stroke in Indonesia reaches 10.9% in population
according to the Indonesia basic health research in
2018 (Riskesdas, 2018). Increasing the number of
stroke survivors make more survivors live with long-
term disability. To manage the impact,
interdisciplinary complex rehabilitation
interventions were required and assumed to
represent the mainstay of post-stroke care.
Optimal functional recovery of stroke is the
ultimate goal of neurorehabilitation after acute
brain injury. Optimizing sensorimotor performance
in functional action is the main goal of
rehabilitation. New brain imaging techniques are
making it clear that the neurological system is
continually remodeling throughout life and after
damage through experience and learning in
response to activity and behavior (Aqueveque et al.,
2017). The potential ability of the brain to readapt
after an injury is known as neuroplasticity, which is
the basic mechanism underlying improvement in
functional outcome after stroke. Therefore, one
important goal of rehabilitation of stroke patients is
the effective use of neuroplasticity for functional
recovery (Winstein CJ et al., 2016).
The type and extent of neural plasticity are task-
specific, highly time-sensitive and strongly
influenced by environmental factors as well as
motivation and attention. The recovery of function
has been shown to depend on the intensity of therapy,
repetition of specified-skilled movement directed
toward the motor deficits and rewarded with
performance-dependent feedback. Specifically, the
exercise should be repetitive, task-specific,
motivating, salient and intensive for neuroplasticity
to occur (Aqueveque et al., 2017),(van Dokkum et
al., 2015).
Evidence accumulated during the past 2 decades
together with recent advances in the field of stroke
recovery clearly shows that the effects of
neurorehabilitation can be enhanced by behavioral
manipulations. Recently, many training-oriented
rehabilitation techniques have been developed,
which allows the increase of independence and
quality of life of the patients and their family
(Aqueveque et al., 2017).
88
Biben, V. and Syahida, J.
Application of Mirror Neuron System in Post Stroke Rehabilitation.
DOI: 10.5220/0009064100880094
In Proceedings of the 11th National Congress and the 18th Annual Scientific Meeting of Indonesian Physical Medicine and Rehabilitation Association (KONAS XI and PIT XVIII PERDOSRI
2019), pages 88-94
ISBN: 978-989-758-409-1
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
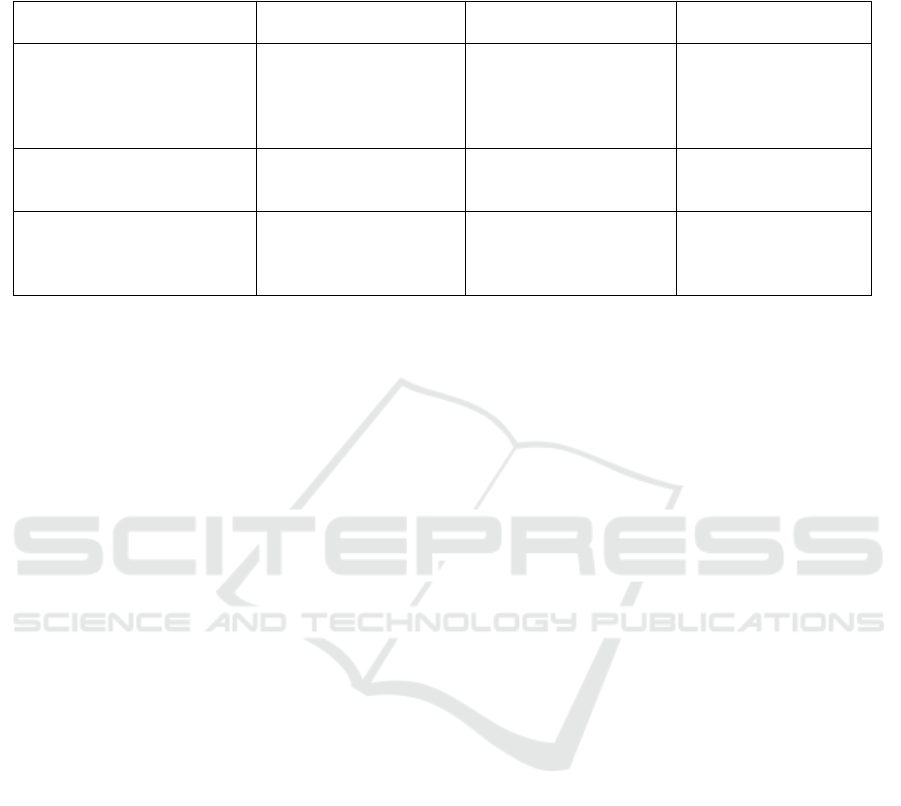
Table 1: Classification according to ICF Model (Kwakkel, 2014).
Body Structure
(i.e., the brain)
Body Function
(i.e., upper limb)
Activity
(a person)
Recovery
Any change in the
structure that leads to
improved function
(includes restitution and
substitution)
Improvement of the
ability to perform a
movement (includes
compensation and
restitution)
Improvement of the
ability to perform a
functional task
(includes compensation
and restitution)
Restitution
Repair: changes toward
the original state
Identical employment of
body components* as
before the injury
Identical task
performance as before
the injury
Compensation/substitution
Alternative employment
of body structures
Alternative employment
of the same body
components as before
injury*
Task performance
using alternative limbs
and/or environmental
adaptations
* A body component is defined as a collection of body structures that contribute to a specific body function
Current resources today are unable to fulfill the
intensity requirement for optimizing post-injury
neuroplasticity, although standard rehabilitation
helps improve motor function after stroke, only
modest benefits have been shown. Limitation of
conventional rehabilitation was including time-
consuming, labor and resource-intensive, dependent
on patient compliance, limited availability
depending on geography, modest and delayed effects
in some patients, requires transportation to special
facilities, initially underappreciated benefits by
stroke survivors and requires costs/insurance
coverage after the initial phase of treatment
(Saposnik et al., 2011).
As a result of the limitations of conventional
rehabilitation, novel strategies targeting motor skill
development and taking advantage of the elements
enhancing experience-dependent plasticity have
recently emerged. In the last 20 years, neuroimaging
techniques and the discovery of mirror neurons
system have brought about a deeper understanding
of brain function, that turn has led to the design of
new treatment approaches such as mirror-symmetric
bimanual movement priming (motor imagery/MI)
and virtual reality (VR) technology (Saposnik et al.,
2011),(García Carrasco and Aboitiz Cantalapiedra,
2016). This article focuses on the review of both
techniques in post-stroke rehabilitation.
2 DISCUSSION
2.1 Motor Recovery Through Cortical
Plastic Reorganization
One of the most important areas affected by stroke is
motor skills. Stroke usually results in injury to the
cerebral cortex, most of the sensory-motor apparatus
in the forebrain including the frontal and parietal
cortex and/or subcortical structures in the striatum
and thalamus, which then produces a deficit of
motor function in the contralateral parts of the body
(Selzer et al., 2014). Improvements in bodily
functions and activities can generally occur
spontaneously or as a result of the learning process.
These include processes of restitution (restoring
damaged nerve tissue function), substitution
(reorganization of nerve pathways to relearn lost
functions), and compensation (new motor patterns
resulting from adaptation or motor substitution
remaining) (table 1).
Weakness and paresis are the most important
impairments in the early stages after stroke as they
lead to learned nonuse of limbs. Immobility, chronic
pain, and some sensory impairments can also
contribute to the learned non-use state. As the
recovery progresses, spasticity and spastic co-
contractions can induce some compensatory
movements, which if are persistent in time and
repeated may contribute to a learned bad use
(Aqueveque et al., 2017).
Understanding the ability of the motor cortex to
carry out functional and structural reorganization is
very important to know because many studies in
experimental animals and humans have shown that
Application of Mirror Neuron System in Post Stroke Rehabilitation
89
the functional and structural motor cortex can be
modified by utilization. The principle of use-
dependent plasticity occurs not only in the brains of
healthy individuals to learn new motor skills but also
in injured brains in re-learning motor skills. To
understand the mechanism of plasticity (the brain's
ability to reorganize by making new neural
connections) post-injury, it is necessary to study the
normal structure and function of the motor cortex
area that functions to control movement.
Rehabilitation approach using either MI or VR
technology was intended to prevent the condition
through a focused and repetitive exercise.
2.2 Mirror Neuron System
Mirror neurons system is a group of specialized
neurons that “mirrors” the actions and behavior of
others. It will discharge both when individuals
perform a given motor act and when they observe
others perform the same motor act (a movement that
has a specific goal). The involvement of MNS is
implicated in neurocognitive functions (social
cognition, language, empathy, the theory of mind)
and neuropsychiatric disorders (Rajmohan and
Mohandas, 2007).
Figure 1: Mirror neuron regions in humans (Rajmohan and
Mohandas, 2007).
Neuroimaging demonstrated the existence of 2
main networks with mirror properties: one residing
in the parietal lobe and the premotor cortex plus the
caudal part of the inferior frontal gyrus
(parietofrontal mirror system), and the other formed
by the insula and the anterior mesial frontal cortex
(limbic mirror system)(figure 1). The parietofrontal
mirror system is involved in the recognition of
voluntary behavior, while the limbic mirror system
is devoted to the recognition of affective behavior
(Cattaneo and Rizzolatti, 2009).
Brain imaging studies reveal that action
observation in humans activates the inferior frontal
gyrus lower part of the precentral gyrus, the rostral
part of the inferior parietal lobule and also the
temporal, occipital and parietal visual areas. The
frontal and the parietal mirror neuron regions are
somatotopically organized. The activation of pars
opercularis of the inferior frontal gyrus reflects the
observation of distal hand and mouth actions,
whereas the activation of the premotor cortex
reflects proximal arm and neck movements. The
mirror neurons will be firing on the frontal and
temporal nodes with an observation of transitive
actions, while that of intransitive (meaningless)
actions result in the firing of the frontal node only.
2.3 Motor Imagery
Various definitions of motor imagery have been
coined by experts. Sharma states that motor imagery
is a dynamic state in which a representation of motor
activity inactivated in memory without any motor
output. Meanwhile, according to Mcavinue, the
concept of motor imagery is a motor representation
or prototype of the movement that is connected with
the memory process. In short, motor imagery can
also be interpreted as “activities to imagine the
movement of the body”(Garcia-Rudolph et al, 2019).
Annett affirmed the importance of volunteer
control of the imagery performers when doing motor
imagery. Two perspectives can be used when
imagining movement, internal perspectives, and
external perspectives. In the internal perspective (or
kinaesthetic), subjects imagine the sensations of
motion in their bodies. External imagery or
perspective was used visual component, in which
subjects imagine seeing themselves from the
viewpoint of an external observer. Therefore, the
activity of imagining a movement from both an
internal or external perspective that involves
manipulating an object can be called motor imagery
(García Carrasco and Aboitiz Cantalapiedra, 2016).
Studies using Transcranial Magnetic Stimulation
(TMS) show an increase in motor-evoked potential
(MEP) amplitude which is a marker of corticospinal
excitability. When motor imagery is performed there
is an increase in MEP amplitude compared to at rest,
MEP amplitude increases only in the muscles
involved in imagined movements and when
performing MI (figure 2). In general, kinesthetic
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
90

imagery activates the cortical motor better than
visual imagery (Ruffino, et al, 2017).
Figure 2: The specificity of corticospinal excitability in
motor imagery. Increased MEP occurs in the flexor carpi
radialis muscle (FCR) and not in the extensor carpi
radialis (ECR) when imagining flexion movements of the
hand (Ruffino et al, 2017).
The observation of other individuals performing
skilled movements, as well as MI was proved
effective for motor training. Neuroimaging studies
have shown that the primary motor cortex (M1) and
secondary motor areas, including the premotor
cortex, supplementary motor area, and the parietal
cortices, are activated during M1 tasks and motor
execution (Lotze et al., 1999). Functional imaging is
used to find out the involvement of primary cortex
motor in motor imagery and compare it with real
movements. Based on several previous studies, there
are different conclusions regarding the involvement
of the motor cortex, especially Broadmann area 4
(BA 4) in motor imagery. In primates and humans,
BA 4 cam be divided into two, BA 4 anterior (BA 4a)
and BA 4 posterior (BA 4p). BA 4a is thought to
have more role in the execution of movements than
produce a real movement. Whereas BA 4b is more
involves in cognitive tasks and non-execution
functions. Besides, BA 4b is also activated by
sensory input and can be modulated by attention.
Because motor imagery does not principally involve
motor execution, it is suspected that activation of
BA 4 when imagery is carried out is more inclined
to BA 4p. However, Sharma's study shows that
activation occurs in both BA 4p and BA 4a when
performing motor imagery with B4p activation
which tends to be stronger when compared to BA 4a.
When compared to the execution of real movements,
these two parts of the BA 4 area are relatively
weaker when doing the motor imagery. Meanwhile,
when viewed from its distribution, the activation of
BA 4 between motor imagery and movement
execution has a similar pattern. Cortex activation
when motor imagery still adheres to the principle of
motor lateralization in the brain similar to the
execution of movements. The things above are proof
of the relationship between the movement being
executed and the motor imagery.
Motor imagery could also divide based on the
motor representation involved whether it is done
consciously (explicit motor imagery) or
unconsciously (implicit motor imagery). The main
difference between explicit and implicit motor
imagery is the level of awareness involved in doing
imagery. Explicit motor imagery can be measured
by an independent questionnaire or by the mental
chronometry paradigm. In this measurement,
subjects are asked to do motor imagery and
consciously imagine their movements. Whereas the
implicit motor imagery measured is the prospective
action decision or the motor perception of the
participants. Participants are asked to make
decisions based on the visual stimulus provided. For
example, participants are asked to choose a picture
of the position of the hand that is most comfortable
holding a log in a certain position. When doing this
task, participants are unconsciously asked to do
motor imagery.
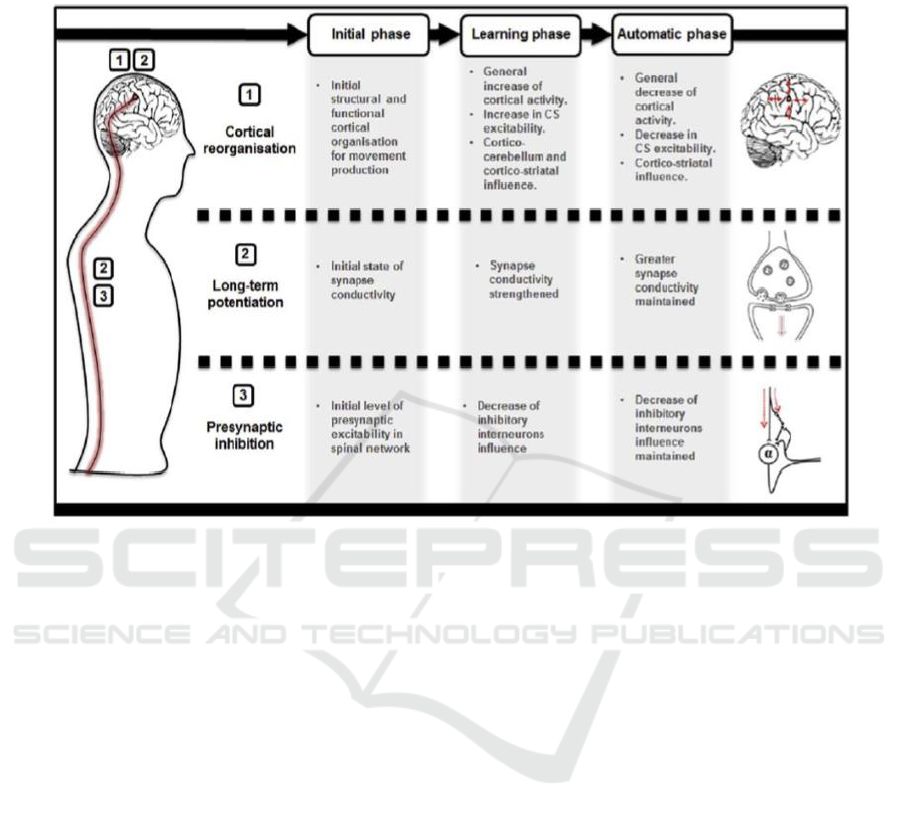
The following is a summary of changes or
adaptations to the central nervous system that are
triggered by motor imagery exercise (figure 3)
(Ruffino et al., 2017):
a. At the cortical level, both cortical mapping that
represents the muscles being trained and the
excitability of the corticospinal pathway will
increase in the first week of exercise.
Furthermore, it will decrease when it reaches
performance stabilization in the automation
phase. In the initial phase of corticocerebellum
tissue and corticostriatal tissue will be activated.
Next, when achieving automation, only
corticostriatal will be activated to recall the
stored motor patterns.
b. At the cortical and spinal level, a long-term
potentiation process can occur and synapses
strengthen. Motor imagery will produce a
subliminal motor signal that will run along the
corticospinal tract until it reaches the structure in
the spinal cord without activating alpha motor
neurons. This subliminal motor signal will play
a role in increasing the sensibility and
conductivity of synapses on the corticospinal
tract.
c. At the spinal level, there is a reduction in
presynaptic inhibition which increases signal
conductivity which is thought to be caused by
descending motor output resulting from motor
imagery exercise.
Application of Mirror Neuron System in Post Stroke Rehabilitation
91
d.
Figure 3: Neural Adaptation Model After Motor Imagery Exercise (Ruffino C, et al, 2017).
2.4 Virtual Reality
Virtual reality (VR) is a computer-based technology
that allows users to interact with the multisensory
simulated environment and receive “real-time”
feedback on performance by computer software and
experienced by the user through a human-machine
interface (Calabrò et al., 2017a). VR is the
stimulation of a real-time environment, scenario or
activity that generated. VR is made using hardware
and software that allows users to interact with
objects and events that appear and sound, and in
some cases can be felt, like those in the real world.
These two environments communicate and exchange
information through a barrier called interface. The
interface can be considered as a translator between
the user and the VR system. The user performs an
action (e.g. movement, speaking) as input, this
interface will translate this action into a digital
signal that can be processed and interpreted by the
system. The system will do a reaction that will be
translated by the interface into something that users
can feel physically (e.g. pictures, sounds, touching
sensations, and so on). Finally, the users will
interpret the information and react to the system. A
stronger sense of “presence” in the virtual world can
be achieved because of different feedback modalities
including visual and audio feedback and less
frequent haptic and vestibular feedback
(Reinkensmeyer DJ et al., 2016).
VR provides the patient with multisensory
feedbacks that can potentiate the use-dependent
plasticity processes within the sensory-motor cortex,
thus promoting or enhancing functional motor
recovery through visuomotor cortical facilitation.
Furthermore, VR can increase patient’s motivation
during rehabilitation by decreasing the perception of
exertion, thus allowing patients to exercise more
effortlessly and regularly (Calabrò et al., 2017b) The
use of an avatar may strengthen the use-dependent
plastic changes within higher sensory-motor areas
belonging to the mirror neuron system (MNS). The
observation of an action, even simulated (on a screen)
allow the recruitment of stored motor programs that
would promote movement execution recovery.
(Modroño et al., 2013) These processes are
expressed by wide changes in
and
oscillation
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
92

magnitude at the electroencephalography (EEG)
across the brain areas putatively belonging to the
MNS (including the inferior frontal gyrus, the lower
part of the precentral gyrus, the rostral part of the
inferior parietal lobule and the temporal, occipital
and parietal visual areas). (Laver et al., 2015)
Broadband involvement may be due to the
recruitment of multiple brain pathways expressing
both bottom-up (automatic recruitment of movement
simulation) and top-down (task-driven) neural
processes within the MNS implicated in locomotion
recognition. Recent work has shown that observed,
executed, and imagined action representations are
decoded from putative mirror neuron areas,
including Broca’s area and ventral premotor cortex,
which have a complex interplay with the traditional
MNS area generating the rhythm (Filimon et al.,
2015).
Training in VR is beneficial for restoring neural
function through several neurophysiological
processes that enhanced the potential for
neuroplastic changes early in the recovery phase and
stimulation of sensorimotor areas that may otherwise
undergo deterioration due to disuse. Many of motor
learning principles that become part of VR in
successfully motor skill development such as
massed repetition practice, task-specific practice,
goal-directed task, and meaningful practice. This
principle boosts the motivation of patients and
serves as a pleasurable experience during treatment
by controlling the level of difficulty and the
variability of the task (Brunner et al., 2014). With
VR, there also a potential mechanism of action that
works in enhancing skill motoric development, such
as augmented feedback that importance in motor
learning. At the behavioral level, movement errors in
the visual domain can influence motor cortical areas
during moor learning and active/rewarded practice.
Feedback can be used to reduce movement errors
and can shape neural activity in motor and premotor
areas. Even observation of actions was done in VR,
if performed repetitiously an intentionally, it can
facilitate the magnitude of motor evoked potential
(MEPs) and influence corticocortical interactions
(both intracortical facilitation and inhibition) in the
motor and premotor areas (Fu et al., 2015).
2.5 Implications For Practice Of VR
Virtual reality-based interventions have been used
for almost 2 decades, but there is still controversy
regarding the efficacy of using virtual reality in
stroke rehabilitation. Cochrane review conducted in
2017 concludes that the use of virtual reality and
interactive video gaming is no more useful in
improving function in the upper limbs when
compared to conventional therapy. Virtual reality
can improve upper limb function and activities of
daily life if used in addition to previous therapies (to
increase therapy time) (Laver KE et al., 2017).
Meanwhile, a study by Maier et al evaluated the
efficacy of 2 types of VR systems, named Specific
VR (SVR) and Non Specific VR (NSVR) with
Conventional Therapy (CT) for rehabilitation of
upper limb function and activity after stroke with the
results showed that SVR is more beneficial than CT
in the recovery of upper limb function, whereas in
the use of NSVR it does not show benefits (Maier M
et al., 2019).
The VR system to train balance and the ability
to walk in post-stroke patients needs special
requirements that require greater technical space
related to patient safety issues. In contrast to VR
therapy in the upper limb which allows the patient to
sit while doing movements with upper extremities,
while for patients who have problems with balance
and walking patterns require patients to walk upright.
Systematic reviews and meta-analysis of randomized
controlled trials conducted by Li et al and de Rooji
et al, showed that VR can improve balance and
ability to walk after a stroke. Llorens et al conducted
a study with the result that exercise with virtual
stepping can improve balance when compared to
conventional therapy. Participants are required to
step on items that appear around the circle with the
nearest foot while maintaining the other foot in the
circle. This intervention also encourages an increase
in walking speed. The system is also used in home-
based intervention with the same results as those
done in the clinic (Maier M et al., 2019). The results
of this study differ from the Cochrane review which
shows that there is not enough evidence about the
effectiveness of virtual reality and interactive video
gaming on walking speed, balance, participation or
quality of life for post-stroke patients (Laver KE et
al., 2017).There is low-quality evidence that VR is a
safe and effective method of improving function and
activities of daily living function following stroke.
Patients in the acute and subacute phases with
milder severity strokes appear to be most likely to
benefit from this technique. However, there is a lack
of information regarding the most effective types of
programs and even whether programs specifically
designed for rehabilitation settings are more
effective than commercial gaming console.
Application of Mirror Neuron System in Post Stroke Rehabilitation
93

3 CONCLUSIONS
MI technique or VR systems can be applied as a
single technique or combination for driving
neuroplasticity and lead to benefits in motor function
improvement after stroke. The use of MI or VR in
post-stroke proved that it can facilitate cortical
reorganization. Future studies need to be done to
determine whether the combination of MI and VR
with also conventional therapy will enhance stroke
rehabilitation.
REFERENCES
Aqueveque P, Ortega P, Pino E, Saavedra F, Germany E,
Gómez B. After Stroke Movement Impairments: A
Review of Current Technologies for Rehabilitation. In:
Uner T, editor. 2017. Physical Disabilities-
Theurapeutic Implications. Turkey:Intechopen;.p.95-
116. Available from : https://doi.org/10.5772/67577
Brunner I, Skouen JS, Hofstad H, Strand LI, Becker F,
Sanders AM, et al. 2014.Virtual reality training for
upper extremity in subacute stroke (VIRTUES): study
protocol for a randomized controlled multicenter trial.
BMC Neurol. 2014 Sep 28;14:186.
Calabrò RS, Naro A, Russo M, Leo A, De Luca R,
Balletta T, et al. 2017. The role of virtual reality in
improving motor performance as revealed by EEG: a
randomized clinical trial. J Neuroeng Rehabil. 2017
Jun 7;(14)1:53.
Calabrò RS, Russo M, Naro A, De Luca R, Leo A,
Tomasello P, et al. 2017. Robotic gait training in
multiple sclerosis rehabilitation: Can virtual reality
make the difference? Findings from a randomized
controlled trial. J Neurol Sci. 2017 Jun 15;377:25–30.
Cattaneo L, Rizzolatti G. 2009. The Mirror Neuron
System. Arch Neurol. 2009 May;66(5):557–560.
de Rooij GLI, Meijer J. 2016. Effect of Virtual Reality
Training on Balance and Gait Ability in Patients with
Stroke: Systematic Review and Meta-Analysis. Phys
Ther.2016 Dec;96(12):1905-18.
Diers M, Kamping S, Kirsch P, Rance M, Bekrater-
Bodmann R, Foell J, et al. 2015. Illusion-related brain
activations: a new virtual reality mirror box system for
use during functional magnetic resonance imaging.
Brain Res. 2015 Jan 12, 1594:173–182.
Filimon F, Rieth CA, Sereno MI, Cottrell GW.2015.
Observed, Executed, and Imagined Action
Representations can be Decoded from Ventral and
Dorsal Areas. Cereb Cortex. 2015 Sep;25(9):3144–58.
García Carrasco D, Aboitiz Cantalapiedra J.2016.
Effectiveness of motor imagery or mental practice in
functional recovery after stroke: a systematic review.
Neurologia. 2016 Jan-Feb;31(1):43–52.
García-Rudolph A., Sánchez-Pinsach D, Salleras EO,
Tormos JM. 2019. Subacute stroke physical
rehabilitation evidence in activities of daily living
outcomes: A systematic review of meta-analyses of
randomized controlled trials. Medicine (Baltimore)
2019 Feb;98(8): e14501.
Kwakkel G, Buma FE, and Selzer ME. 2014.
Understanding the mechanisms underlying recovery
after stroke. In Selzer ME, Clarke S, Cohen LG,
Kwakkel G, Miller RH., Textbook of Neural Repair
and Rehabilitation Vol 2(2). Cambridge University
Press; 2014 .p.7-24.
Laver K, George S, Thomas S, Deutsch JE, Crotty
M.2015.Virtual reality for stroke rehabilitation: an
abridged version of a Cochrane review. Eur J Phys
Rehabil Med, 2015 Aug;51(4):497–506.
Laver KE, Lange B, George S, Deutsch JE, Saposnik G,
Crotty M.2017.Virtual reality for stroke rehabilitation.
Cochrane Database Syst Rev. 2017;11: CD008349.
Maier M, Rubio BB, Duff A, Duarte OE, Verschure
P.2019. Effect of Specific Over Nonspecific VR-
Based Rehabilitation on Poststroke Motor Recovery:
A Systematic Meta-analysis. Neurorehabil Neural
Repair. 2019 Feb;33(2):112-29.
Modroño C, Navarrete G, Rodríguez-Hernández AF,
González-Mora JL.2013.Activation of the human
mirror neuron system during the observation of the
manipulation of virtual tools in the absence of a visible
effector limb. Neurosci Lett. 2013 Oct 25;555:220-4.
Prochnow D, Bermúdez i Badia S, Schmidt J, Duff A,
Brunheim S, Kleiser R.2013. A functional magnetic
resonance imaging study of visuomotor processing in
a virtual reality-based paradigm: Rehabilitation
Gaming System. Eur J Neurosci. 2013
May;37(9):1441–7.
Reinkensmeyer DJ, Dietz V.2017. Neurorehabilitation
Technology: Springer International Publishing; 2016.
Ruffino C, Papaxanthis C, Lebon F.2017. Neural plasticity
during motor learning with motor imagery practice:
Review and perspectives. Neuroscience. 2017 Jan
26;341:61-78.
Saposnik G, Levin M, Outcome Research Canada
(SORCan) Working Group.2011. Virtual reality in
stroke rehabilitation: a meta-analysis and implications
for clinicians. Stroke. 2011 May;42(5):1380–6.
Selzer M, Clarke S, Cohen L, Kwakkel G, Miller R.2014.
Textbook of Neural Repair and Rehabilitation Vol 1:
Neural Repair and Plasticity: Cambridge University
Press; 2014.
van Dokkum LEH, Ward T, Laffont I.2015.Brain-
computer interfaces for neurorehabilitation – its
current status as a rehabilitation strategy post-stroke.
Ann Phys Rehabil Med. 2015 Feb;58(1):3-8.
Winstein CJ, Stein J, Arena R, Bates B, Cherney L,
Cramer SC, et al.2016. Guidelines for Adult Stroke
Rehabilitation and Recovery. Stroke. 2016
Jun;47(6):e98-e169.
KONAS XI and PIT XVIII PERDOSRI 2019 - The 11th National Congress and The 18th Annual Scientific Meeting of Indonesian Physical
Medicine and Rehabilitation Association
94
