
X-Ray Diffraction and Morphology Studies of Sulfonated Polystyrene
and Maleated Natural Rubber Blend with PE-g-MA as
Compatibilished
Ahmad Nasir Pulungan
1,3
, Basuki Wirjosentono
2
, Eddiyanto
2,3
and Sunit Hendrana
4
1
Chemistry Postgraduate Study Programs, Universitas Sumatera Utara, Medan 20155, Indonesia
2
Department of Chemistry, Universitas Negeri Medan, Jl. Willem Iskandar Psr. V Medan Estate 20371, Indonesia
3
Department of Chemistry, Universitas Sumatera Utara, Medan 20155, Indonesia
4
Research Centre for Chemistry Indonesian Institute of Sciences,Kompleks Puspitek 452, Serpong, Banten 15314, Indonesia
Keywords: Sulfonated Polystyrene, Maleated Natural Rubber, Blend, X-Ray Diffraction, Morphology.
Abstract: The preparation of blend of sulfonated polystyrene (sPS) and maleated natural rubber (MNR) using the
Polyethylene-graft-maleicanhydride (PE-g-MA) as compatibilished has been done. The blending process
was done in dilute solution in each polymer, followed a steam and casting process at particular
temperatures. The composition of sPS and MNR was varied at 1:9; 2:8; 4:6 and 6:4 (w/w). The
characterization of membrane blend of sPS-MNR/PE-g-MA properties was determined by using X-ray
diffraction (XRD) and scanning electron microscopy (SEM). The data showed that the homogeneity and the
crystallinity of membrane were affected by the composition and process condition. The XRD data show that
the Membrane (sPS-MNR / PE-g-MA) with sPS: MNR ratio 2:8 produces diffraction patterns with higher
intensity and SEM images showed that membrane has a better homogeneous surface morphology than the
others.
1 INTRODUCTION
The formation of polymer blends generally aims to
produce new materials with superior properties
compared to each of the forming polymer materials
(Jhons and Rao, 2019). This method is widely
developed because of the low cost of processing and
short processing time to produce new polymeric
materials. The nature of the polymer blend is
determined mainly by the compatibility of the
polymer of the composition of the mixture, the
process parameters and the desired application
(Favis, BD, & Chalifoux, JP (1988). Polystyrene
(PS) is included in the thermoplastic group with an
extensive application, due to unique properties such
as transparent, high chemical stability, ease of
colouring and low cost (Ebewele, 2000), but the PS
also has limitations such as its fragile nature, low
heat deflection temperature and weak UV resistance
(Ozden, 2004). However, currently sulfonated
polystyrene (sPS) has been widely developed and
used as an electrolyte polymer membrane as an
alternative to the replacement of Nafion membrane
(Hendrana et al., 2016; Mulijani et al., 2014; Lee et
al., 2008), because it has a price conductivity
approaches Nafion. Therefore, the sPS membranes
require other polymeric materials to improve their
mechanical properties (elasticity).
Natural polymeric materials such as natural
rubber (NR) exhibit the best physical properties with
comprehensive industrial applications (Jhons and
Rao, 2008; Sanguansap et al., 2004). NR has
superior physical properties such as firmness, tensile
strength and high elasticity, abrasion and tear
resistance as well as good stickiness and are easy to
grind (Arroyo et al., 2007; Peng et al., 2007 and
Derouet et al., 2009). Therefore, these attracts many
researchers to produce various products for
industrial processes (Wirjosentono et al., 2018).
In this study, the sPS will be blended with the
NR, with the aim to improve the mechanical
properties (elasticity) of the membrane.
Compatibility is improved by modifying the NR to
form a maleated natural rubber (MNR) by grafting
maleic anhydride (MA) into the NR structure.
Nakason et al., (2004) studied grafting of MA onto
natural rubber. The use of maleic anhydride (MA) as
324
Pulungan, A., Wirjosentono, B., Eddiyanto, . and Hendrana, S.
X-Ray Diffraction and Morphology Studies of Sulfonated Polystyrene and Maleated Natural Rubber Blend with PE-g-MA as Compatibilished.
DOI: 10.5220/0008935003240328
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 324-328
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
a coupling agent increases the adhesion and interface
properties of polymer composites (Cao et al., 2012;
Machado, 2000). Ismail et al., (2002), Wirjosentono
et al., (2008) and Wirjosentono et al., (2018)
reported an increase in adhesion of composite
Bamboo fibre with natural rubber; polypropylene
with cyclic natural rubber; and Oil palm empty fruit
bunch with polypropylene.
Preparation of polymer blend from the sPS and
MNR with PE-g-MA as compatibilities is expected
to produce a stable and homogeneous membrane
blend product. Thus, the membrane can be applied
as a proton exchange membrane. Therefore, it is
essential to investigate the properties of the polymer
blend. In this paper, an initial characterization of the
sPS-MNR / PE-g-MA blend by X-ray (XRD)
diffraction and scanning electron microscope (SEM)
will be carried out.
2 MATERIALS AND METHODS
2.1 Materials
Sulfonated polystyrene (sPS) were prepared
(Hendrana, 2013). High-ammonia concentrate
natural rubber (HANR) latex DRC 60% is from PT
IKN Medan-Indonesia. MNR were prepared
according to Pulungan et al. (2017). PE-g-MA is
from Aldrich (~0.5 wt. %). Methanol and toluene are
p.a., grade and all use from Merck.
2.2 Preparation of Membrane Blend of
sPS-MNR/PE-g-MA First Section
The blending process of sPS, MNR and PE-g-MA
polymer materials has been carried out in a very
dilute solution system, followed by a steam and
casting process at particular temperatures.
Comparison of sPS and MNR varied each: 1:90; 2:8;
4:6 and 6:4 (w/w) with the concentration of PE-g-
MA is 3% (w/w). In the initial stages the sPS were
dissolved in toluene: methanol with a ratio of 9:1 at
45
o
C and slowly stirred with a magnetic stirrer. The
MNR and the PE-g-MA were also dissolved in
toluene solvent and slowly stirred with a magnetic
stirrer at 45
o
C. The PE-g-MA solution was added to
the MNR solution, stirred until homogeneous.
Furthermore, the sPS solution was added slowly and
followed by the stirring process with a magnetic
stirrer at 45
o
C for 8 hours. The resulting blend
solution was cast and dried in an oven gear at 45
o
C
for 24 hours. The membrane obtained was dried in a
drying oven at 60°C for 8 hours.
2.3 Characterization of Membrane
Blend of sPS-MNR/PE-g-MA
The initial characterization of membrane blend
includes XRD analysis and morphology with SEM
as follows:
2.3.1 X-Ray Diffraction Studies
The XRD patterns of the membrane were recorded
with an X-ray diffractometer Shimadzu 6100, using
Cu Kα radiation, with a scanning rate of 2
o
min-1 in
a range from 7
o
to 70
o
(2θ). The operating voltage
and current of the tube kept at 40 kV and 30 mA
respectively.
2.3.2 Morphology Analysis
SEM analysis was performed to investigate the
morphology and homogeneity of the membrane
blend. The SEM images were obtained from SEM of
JSM-6510 LA. The surface of the membrane was
coating with gold before being measured under the
microscope.
3 RESULTS AND DISCUSSION
3.1 X-Ray Diffraction Studies
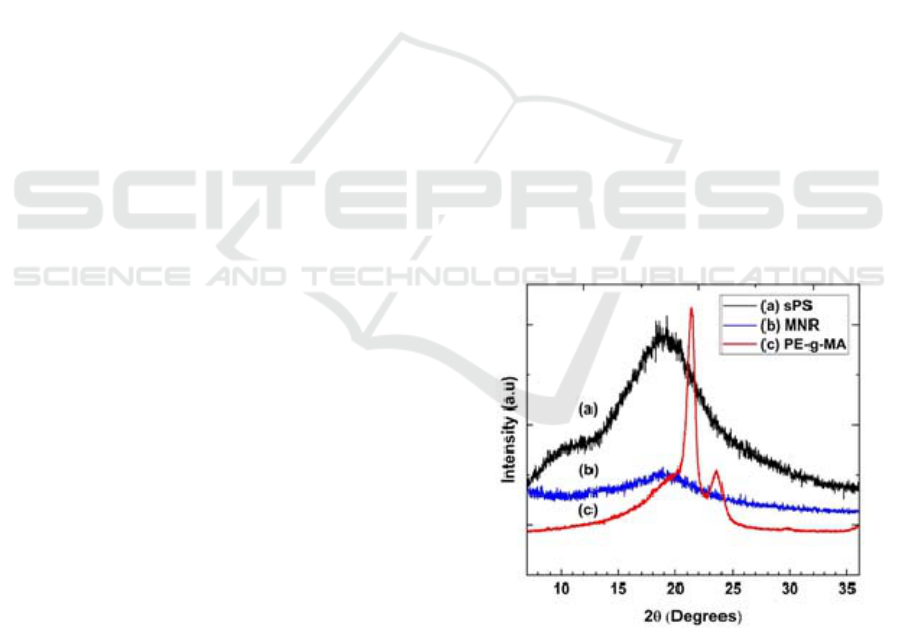
Figure 1: XRD diffractogram of (a) sPS, (b) MNR and (c)
PE-G-MA.
The XRD diffractograms of sPS, MNR and PE-g-
MA are depicted in Figure 1. The XRD
diffractograms crystalline polymers produce sharp
peaks, whereas amorphous polymers will produce
widening peaks. Figure 1 (a) shows the crystalline
X-Ray Diffraction and Morphology Studies of Sulfonated Polystyrene and Maleated Natural Rubber Blend with PE-g-MA as
Compatibilished
325
phase of PE-g-MA with a sharp peak intensity at 2θ
around 21.5
o
. While the sPS diffractogram shows an
amorphous phase with a peak widening at 2θ about
19.4
o
and the MNR shows a more amorphous phase
than the sPS at 2θ around 19.4
o
. Martin et al. (2003)
were reported that the sPS has amorphous properties
because the pure PS polymer is initially amorphous.
The sPS has a peak at 2θ around 19.8
o
and NR has
a peak at 2θ around 19
o
(Jhon and Rao, 2008).
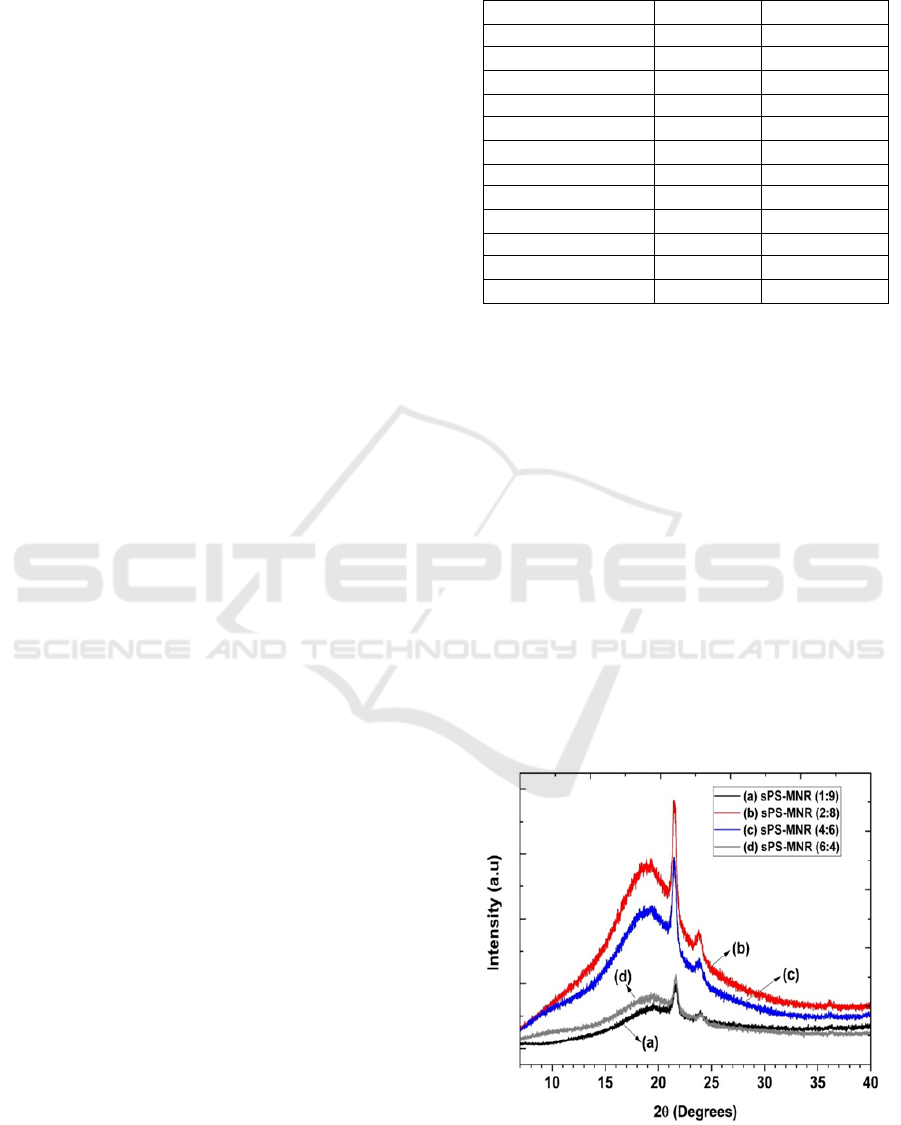
The XRD diffractogram in Figure 2 provides
information about the structure of the polymer blend
from the sPS-MNR/PE-g-MA. The figure shows the
existence of a randomly mixed crystalline and
amorphous state, which illustrates the combined
characteristic peaks of MNR, sPS and PE-g-MA.
The sPS-MNR/PE-g-MA membrane with the
composition of sPS:MNR 2:8 produces the highest
intensity of the main peaks. While the sPS-
MNR/PE-g-MA membrane with the composition of
sPS:MNR 4:6 and 6:4 shows a decrease in the
intensity of the peak in the membrane. This shows
that the ratio of the composition of the membrane
blend gives an influence on the change in the
crystallinity phase. With increasing content of sPS,
the overall crystallinity of membrane blend
decreases. In the polymer material, if the degree of
crystallinity is getting smaller, the elasticity is
getting bigger, this is caused by the branch chain
that occurs, which causes the polymer material to be
more elastic.
To investigate the effect of membrane
composition, the interaction between the sPS and
MNR on the membrane is observed based on the
crystal size change approach. The X-ray diffraction
scattering pattern can provide information about the
configuration of the chain in crystallites, the
estimated crystallite size and the comparison of the
crystalline region with the amorphous region (degree
of crystallinity) of the polymer material. Crystal size
can be determined through the approach of the
FWHM value (full width at half maximum) using
the Debye-Scherrer equation (Kumar and Raji,
2011) as follows:
D = 0.9 λ / B Cos θ (1)
Where:
D = Average Size Crystal
λ = wavelength of XRD (0.15406 nm)
θ = Braag Diffraction angle
B = FWHM value in radian
The results of the calculation of the crystal size of
each membrane are summarized in Table 1.
Table 1: The Average Size Crystal of membrane
sPS-MNR/PE-g-MA.
2θ (º) D (nm)
sPS:MNR (1:9) 19.49 1.49
21.59 5.37
23.97 4.82
sPS:MNR (2:8) 19.31 2.13
21.49 6.97
23.82 3.85
sPS:MNR (4:6) 19.37 1.54
21.49 4.76
23.82 5.12
sPS:MNR
(
6:4
)
19.32 1.65
21.63 5.46
23.86 3.52
Based on the data in table 1, it can be seen that
an increase in the sPS concentration to the sPS:
MNR (2:8) increases the crystal grain size at 2θ
around 19.4
o
but the crystal grain size gets smaller at
the sPS:MNR ratio of 4:6 and 6:4. This is because in
the sPS:MNR 2:8 the diffraction peak width is
narrower so that the half peak width value (FWHM)
is smaller, and the crystal grain size is increasing.
However, at the sPS: MNR 4:6 and 6:4, the FWHM
value is enlarge which shows that the width of the
diffraction peak widened as well due to the
increased concentration of sPS in the membrane.
These data indicate that the effective interaction of
the polymer blend is at the ratio of SPS: MNR 2:8
(w/w). The higher number of sPS actually disturbs
the effectiveness of the interaction between sPS and
MNR. This resulted in interactions that occurred
between sPSS and NMR being dominated by
sulfonate groups from sPS.
Figure 2: XRD diffractogram of sPS-MNR/PE-g-MA
membrane with sPS: MNR composition: (a) 1: 9; (b) 2: 8;
(c) 4: 6 and (d) 6: 4 (w/w) respectively.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
326

3.2 Morphological Measurement using
SEM
The surface morphology of each membrane is given
in Figure 3.
Figure 3: SEM images of sPS-MNR/PE-g-MA membrane
with sPS: MNR composition ratio (a) 1: 9; (b) 2: 8; (c) 4:
6 and (d) 6: 4 (w /w).
In Figure 3, it can be seen that the sPS-
MNR/PE-g-MA membrane with a sPS:MNR 2:8
composition ratio shows a more homogeneous
surface compared to other membranes. In
membranes with a composition ratio of sPS:MNR
2:8 produces a dense and homogeneous surface even
though the presence of grainy components of the
sPS is not completely dissolved with the MNR. This
is due to the increase in adhesion between the sPS
and MNR. While on membranes with a greater sPS
composition ratio, i.e. on the sPS: MNR 4:6 and 6:4
membranes, it appears that the presence of grainy
components from sPS forms larger sPS cluster
groups as a result of the increasing number of sPS
that are not completely dissolved with the MNR.
From this data, information is obtained that the
optimum blending composition is produced at the
sPS:MNR ratio = 2:8 (w/w). This is due to the
reduced adhesion force because the interactions that
occur are dominated by sulfonate groups from sPS.
Increased sPS concentrations do not provide better
membrane compatibility. Concentration plays a role
but not a high concentration of Sps.
4 CONCLUSIONS
Based on XRD data, membranes with the
composition of sPS:MNR with ratio 2:8 produce the
highest peak intensity diffractogram, as a result of
the most effective interaction between sPS and
MNR. This is also supported by SEM of the
membrane surface with a more homogeneous
surface morphology compared to that of the
membrane surface with the composition of sPS:
MNR 4:6; 6:4 and 1:9 respectively. The composition
ratio plays a role to produce compatible sPS-MNR /
PE-g-MA membrane.
ACKNOWLEDGEMENTS
The authors would like to thank University of
Sumatera Utara and Universitas Negeri Medan for
funding this work through research program of
“Penelitian Disertasi Doktor tahun 2017”, also to
P2F LIPI, Chemistry Laboratory of The Department
of Chemistry and Material Laboratory of The
Department of physics, Universitas Negeri Medan
for providing facilities of the work.
REFERENCES
Arroyo, M., Lopez-Manchado, M. A., Valentin, J. L., and
Carretero, J. 2007. Morphology/behaviour relationship
of nanocomposites based on natural rubber/epoxidized
natural rubber blends. Composites science and
technology, 67(7), 1330-1339.
Cao, X. V., Ismail, H., Rashid, A. A., Takeichi, T., and
Vo-Huu, T. 2012. Maleated natural rubber as a
coupling agent for recycled high density
polyethylene/natural rubber/kenaf powder
biocomposites. Polymer-Plastics Technology and
Engineering, 51(9), 904-910.
Derouet, D., Intharapat, P., Tran, Q. N., Gohier, F., and
Nakason, C. 2009. Graft copolymers of natural rubber
and poly (dimethyl (acryloyloxymethyl) phosphonate)
(NR-g-PDMAMP) or poly (dimethyl
(methacryloyloxyethyl) phosphonate) (NR-g-
PDMMEP) from photopolymerization in latex
medium. European Polymer Journal, 45(3), 820-836.
Ebewele, R. O. 2000. Polymer science and technology.
CRC press.
Favis, B. D., and Chalifoux, J. P. 1988. Influence of
composition on the morphology of
polypropylene/polycarbonate blends. Polymer, 29(10),
1761-1767.
Hendrana, S., Pudjiastuti, S., Chaldun, E. R., Widodo, H.,
Rochliadi, A., and Handono, M. A. 2016. Role of
thickness and density on the ionic conductivity of fuel
X-Ray Diffraction and Morphology Studies of Sulfonated Polystyrene and Maleated Natural Rubber Blend with PE-g-MA as
Compatibilished
327

cell membrane prepared with supramolecular
structure. In A. Rusydi, D. H. Wicaksono, T.
Anggono, Y. Herbani, & F. Febriani (Eds.), AIP
Conference Proceedings, 1711(1).
Hendrana, S., Chaldun, E. R., Pudjiastuti, S., Rahayu, I.,
Natanael, C. L., Oktaverina, D., & Semboor, M. S.
2013. Heterogeneous Sulphonation of Polystyrene for
Polymer Electrolyte Membrane Fuel Cell Application.
In Macromolecular Symposia, 327(1), 80-84.
Ismail, H., Edyham, M. R., and Wirjosentono, B. 2002.
Bamboo fibre filled natural rubber composites: the
effects of filler loading and bonding agent. Polymer
testing, 21(2), 139-144.
Johns, J., and Rao, V. 2009. Thermal stability,
morphology, and X-ray diffraction studies of
dynamically vulcanized natural rubber/chitosan
blends. Journal of materials science, 44(15), 4087-
4094.
Kumar, K. B., and Raji, P. 2011. Synthesis and
characterization of nano zinc oxide by sol gel spin
coating. Recent research in science and
technology, 3(3).
Lee, W., Kim, H., and Lee, H. 2008. Proton exchange
membrane using partially sulfonated polystyrene-b-
poly (dimethylsiloxane) for direct methanol fuel cell.
Journal of Membrane Science, 320(1), 78-85.
Machado, A.V., Van Duin, M. and Covas, J.A., 2000.
Monitoring polyolefin modification along the axis of a
twin‐screw extruder. II. Maleic anhydride grafting.
Journal of Polymer Science part A: Polymer
Chemistry, 38(21), pp.3919-3932.
Martins, C. R., Ruggeri, G., and De Paoli, M. A. 2003.
Synthesis in pilot plant scale and physical properties
of sulfonated polystyrene. Journal of the Brazilian
Chemical Society, 14(5), 797-802.
Mulijani, S., Dahlan, K., & Wulanawati, A. 2014.
Sulfonated polystyrene copolymer: synthesis,
characterization and its application of membrane for
direct methanol fuel cell (DMFC). Int J Mater, Mech
Manuf, 2, 36-40.
Nakason, C., Kaesaman, A., and Supasanthitikul, P.
(2004). The grafting of maleic anhydride onto natural
rubber. Polymer Testing, 23(1), 35-41.
Özden, G. Ü. L. S. Ü. M. 2004. Synthesis and
Characterization of Polystyrene Clay Nanocomposites.
Chemical Engineering.
Peng, Z., Kong, L. X., Li, S. D., Chen, Y., and Huang, M.
F. 2007. Self-assembled natural rubber/silica
nanocomposites: its preparation and characterization.
Composites Science and Technology, 67(15), 3130-
3139.
Pulungan, A. N., Wirjosentono, B., and Eddiyanto, S. H.
Grafting Maleat Anhidrida Pada Lateks Karet Alam
Dengan Inisiator Benzoil Peroksida. 2017. Prosiding
Seminar Hilirisasi Penelitian Untuk Kesejahteraan
Masyarakat Lembaga Penelitian Universitas Negeri
Medan.
Sanguansap, K., Suteewong, T., Saendee, P.,
Buranabunya, U., and Tangboriboonrat, P. 2005.
Composite natural rubber based latex particles: a novel
approach. Polymer, 46(4), 1373-1378.
Suryanarayana, C., and Grant, N. 1998. A Practical
Approach. Plenum Press. New York, 4, 513-515.
Wirjosentono, B., Guritno, P., and Ismail, H. 2004. Oil
palm empty fruit bunch filled polypropylene
composites. International Journal of Polymeric
Materials, 53(4), 295-306.
Wirjosentono, B., Siregar, A. H., Nasution, T. I.,
Dalimunthe, K. Z., and Nasution, D. A. 2018.
Compatibilitation of cyclic natural rubber (resiprene-
35) with polypropylene in the presence of oleic acid
and benzoyl peroxide. In Journal of Physics:
Conference Series, IOP Publishing, 1116(4).
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
328
