
Modification of Pulp Cellulose of Belangke Bamboo (Gigantochloa
pruriens) using [2-(Acryloyloxy)Ethyl] Trimethyl Ammonium
Chloride and Maleic Anhydride
Rina Ridara
1
, Diana Adnanda Nasution
2
and Basuki Wirjosentono
2*
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jl. Bioteknologi
No. 1, Medan 20155, Indonesia
Keywords: Cellulose, Belangke Bamboo, Modification, Antimicrobial Compound.
Abstract: In this work, pulp cellulose (Cell) was prepared from Belangke bamboo (gigantochloa pruriens) by Craft
delignification process. The AETAC/MA-modified Cellulose (AETAC/MA-g-Cell) was characterised using
infrared spectroscopy (FTIR) for chemical structure, differential scanning calorimetry (DSC) for thermal
properties and scanning electron microscopy (SEM) for morphological images. Results of FTIR spectra of
the AETAC/MA-g-Cell after exhaustive Sokhlet extraction in n-hexane still showed stable absorption peak
of AETAC/MA carbonyl group (>C=O) at 1705 cm
-1
and disappearance of double bond absorption peak af
acryloyl group (>C=C<) at 1630 cm
-1
. These evidences indicated that the AETAC/MA modifiers have
successfully bound into the cellulose, in which hydroxyl groups of the cellulose have esterified with maleic
anhydride and bound with acryloyl groups of AETAC. Further data of DSC analysis of the modified
cellulose showed slightly lower decomposition temperature of 300
o
C when compared to that of fresh
cellulose of 270-400
o
C. Whereas SEM images of the modified cellulose also indicated rougher surface
when compared to that of fresh cellulose fibres. The AETAC/MA-modified cellulose then may be utilised
as antimicrobial materials for various cellulose products.
1 INTRODUCTION
Indonesia is a tropical country rich in non-timber
crops that can be used as an alternative raw material
for the pulp and paper industry. One type of non-
timber plant is bamboo. Bamboo is a general term
for members of the wooden grass, the Bambusoideae
subfamily and the Andropogoneae / Poacea family.
Bamboo has several advantages compared to woody
plants that grow fast and can be harvested after 3-5
years of planting, much shorter than needle wood
which takes 10-20 years. In addition, bamboo has
high productivity and can grow in arid soils (My &
Le, 2015). As a non woody plant, bamboo is known
for its long fiber with an average fiber length of
1.90-3.24 (Tian, 2013).
Bamboo is grass, cylindrical in shape which is
mostly hollow (though some species are solid
cylindrical). Bamboo usually has a height of 20-25
meters. Biomass production in the planting season is
around 3-5 months. Bamboo consists of 26-43%
cellulose, 21-31% lignin, and 15-26% hemicellulose.
In theory, the mechanical properties of bamboo
mainly depend on (1) species, (2) age, (3) moisture
content, (4) position along the stem (top or bottom),
and (5) node and segment position. Belangke
bamboo (Gigantochloa pruriens) which is 125
species of bamboo in Indonesia can grow to 10
meters in length with a diameter of 5 cm and a
length of 35 cm. This type of bamboo has been used
by the community for various household, equipment,
construction and handicraft industries. Textile fibers
made from bamboo are still not popular, and even
more than 1500 species of bamboo in the world,
only a few types are processed into textile materials
(Waite, 2009).
However, given its availability, especially in
Indonesia which is abundant, the prospect of
bamboo fiber as a textile material for clothing is
quite promising compared to other natural fibers.
Nowadays, the use of antimicrobial textile materials
has been growing, due to the people's perception of
Ridara, R., Adnanda Nasution, D. and Wirjosentono, B.
Modification of Pulp Cellulose of Belangke Bamboo (Gigantochloa pruriens) using [2-(Acryloyloxy)Ethyl] Trimethyl Ammonium Chloride and Maleic Anhydride.
DOI: 10.5220/0008932303050311
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 305-311
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
305

using hygienic and clean textile materials which is
increasing. Textile materials, especially for
underwear, are indeed susceptible to exposure, and
can even be a living medium for microbes, which
cause these textile materials: easy to smell, have
spots, and damage (Kumar, 2011).
Cellulose is one of the most widely dispersed,
renewable, and biodegradable polymers. Cellulose is
a natural polymer that does not dissolve in water due
to long chains and high molecular weight (more than
500,000 Da) (Fathanah, Lubis, Othman, Handayani,
& Karlina, 2017).
Cellulose is the main element of all plant material,
forming about half to one third of all plant tissue and
is constantly formed from photosynthesis (Sun, Sun,
Zhao, & Sun, 2004). This is the main structural
component that provides strength and stability to plant
cell walls which are arranged in microfibrils in cell
walls, interrupted by hemicellulose and surrounded by
a lignin matrix. Depending on the type of plant, most
plant material consists of about 40 - 55% cellulose, 15
- 35% lignin and 25 - 40% hemicellulose. On the cell
wall of natural plants, cellulose crystals are covered
with these substances which makes it difficult to get
pure cellulose. Chemically, cellulose is a naturally
occurring linear polymer of anhydroglucose unit
connected to one and four carbon atoms by β-
glycosidic bonds. It is proven by the presence of three
hydroxyl (OH) groups with different acidity /
reactivity, where secondary OH is located in the C-2,
and C-3 positions, and the primary OH is located in
the C-6 position. This is also verified by the formation
of various strong hydrogen bonds between molecules
and intramolecules (Penjumras, Abdul, Talib, &
Abdan, 2014). In recent years, interest in cellulose-
based materials has increased due to the demand for
renewable resources (Mohanty et al., 2005).
MA grafting into various polymers has been
popular for the past 20 years. Grafted copolymers
can be used as the main component. Various
polymers have been used as backbones for grafting
maleic anhydride. Other types of thermoplastics (PS,
EVA, PES), as well as elastomers (EPDM, EPR,
NR), can also be used as polymers for MA grafting
(Krump, Alexy, & Luyt, 2005).
As is known, 2-acryloyloxyethyl trimethyl
ammonium chloride (AETAC) consists of
quaternary ammonium salt groups and unsaturated
vinyl groups. Vinyl groups can be polymerized and
can react with a variety of vinyl monomers, which in
2016 Shen et al conducted research on poly [(2-
acryloyloxyethyl trimethyl ammonium chloride) -co-
(acrylic acid)] branches onto starch for cotton warp
sizing (Shen, Zhu, & Liu, 2016).
Wool fiber antimicrobial textile material has
been developed using a coating technique with a
bioactive polymer material, [2- (acryloiloxy) ethyl]
trimethylammonium chloride (AETAC).
Antimicrobial properties of wool fiber coated with
bioactive polymers were tested against Gram-
positive and Gram-negative bacteria,
Staphylococcus aureus and Klebsiella pneumoniae.
Furthermore, the surface morphology characteristics
of the fibers were tested using reflected infrared
spectroscopy (ATR-FTIR), electron microscopy
(SEM), tensile strength and contact angle to water
drops. the result is that Q-chitosan-based
antibacterial properties against wool can be
improved by grafting wool fabric with polystyrene
sulphonate (pSS), which shows very good
antibacterial activity against Staphylococcus aureus
and Klebsiella but less good against the fungus
Aspergillus fumigatus. Transplanting a wool fabric
with pSS not only increases the tensile strength of
the fabric but also increases the durability of the
treatment of several washings. pSS-g-wool, Q-
chitosan evenly covers the surface of the treated
fiber and after 5x the washing is still intact.
Therefore this grafting process is commercially
viable for wool fabrics (Hassan, 2015).
The two most common methods used to analyze
thermal properties are thermogravimetric analysis
(TGA) and (DSC). The TGA technique measures the
weight loss and mass change of a substance as a
function of temperature. However, there are several
reactions that can occur without mass loss. In this
case, DSC is able to detect these reactions
(Zakikhani, 2016).
In this research, the surface modification of
cellulose fiber from Belangke bamboo will be
modified with antimicrobial compound (AETAC),
with the help of maleic anhydride (AM) and
ammonium persulfate as comonomer and initiator.
Furthermore, the surface morphology characteristics
of the fibers were tested using reflected infrared
spectroscopy (ATR-FTIR), electron microscopy
(SEM), Thermal Analysis Differential Scanning
Calorimetry (DSC).
2 MATERIALS AND METHODS
2.1 Materials
The material used in this study is 80% solution of 2-
(acryloyloxy)-ethyltrimethylammonium chloride were
purchased from Sigma-Aldrich Chemicals (USA) and
used without any purification, ammonium persulfate
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
306

(NH
4
)
2
SO
4
≥90% from China, maleic anhydride with
weight molecul 98.06 g/mol, acetone and toluene
from Mallinckrodt Chemicals (USA). Belangke
bamboo that is used comes from Pangkalan Brandan,
Sei Lepan Subdistrict, Langkat Regency, North
Sumatra, located on the east coast of the island of
Sumatra, about 60 km north of Binjai City.
2.2 Methods
2.2.1 Preparation of Belangke Bamboo
The bamboo Belangke obtained is washed with
water until clean then dried in the sun to dry then cut
with a grinding cutter until it becomes powder.
2.2.2 Isolation of α-cellulose from Belangke
Bamboo
As much as 75 grams of Belangke Bamboo Powder
then put into a glass beaker and added 1 L mixture
of 3.5% HNO
3
and 10 mg NaNO
2
heated on a hot
plate at 90
o
C for 2 hours. After that filtered and
washed pulp until neutral filtrate. Then added with
750 ml of solution containing 2% of NaOH and 2%
of Na
2
SO
3
at 50
o
C for 1 h then filtered and washed
pulp until neutral filtrate. Then bleaching with 500
ml of 1.75% of NaOCl solution at boiling
temperature for 30 minutes. The pulp is filtered and
washed until neutral filtrate. After that, α-cellulose
was purified from a sample of 500 ml of 17.5%
NaOH solution at 80
o
C for 0.5 hours then filtered,
washed until neutral filtrate. Continuing bleaching
with 10% H
2
O
2
at 60
o
C for 15 minutes. Washed and
filtered cellulose to neutral. Oven drying was carried
out for 3 hours at 60
o
C and stored in a desiccator
(Ohwoavworhua, 2005). Characterization of α-
cellulose produced includes: % yield, chemical-
physical properties analysis, functional groups
(FTIR), morphology (SEM), thermal analysis
(DSC).
2.2.3 Modification of α-cellulose with Maleic
Anhydride
Modification of α-cellulose bamboo belangke with
maleic anhydride was done by reflux for 2 hours
using toluene as a medium. Reaction optimization
was carried out with fixed cellulose levels (100 phr)
with maleic anhydride variations (5, 10, 15, 20 phr).
The toluene solvent was evaporated at 110
o
C,
then washed with acetone to remove free maleate.
The result of cellulose / AM is dried in an oven at
70
o
C until the weight remains, then characterized by
functional group analysis (FTIR).
2.2.4 Modification of Cellulose Fibers
with [2-(Acryloiloxy) Ethyl]
Trimethyl Ammonium Chloride
(AETAC)
Surface modification of Belangke bamboo cellulose
fibers with [2- (acryloyloxy) ethyl]
trimethylammonium chloride (AETAC) 80% was
carried out in the reflux phase modification reactor
and stirring with toluene solvent and the addition of
maleic anhydride (AM) and ammonium persulfate as
comonomers and initiators. Reaction optimization
was carried out by varying the levels of maleic
anhydride and AETAC reagent levels by adding
10% ammonium persulfate from the optimum level
of maleic anhydride. The toluene solvent was taken
back by a vacuum evaporator and the AM-AETAC-
modified cellulose was dried in a 70
o
C vacuum oven
to a fixed weight. Characterization of α-cellulose
modified with [2- (acryloyloxy) ethyl]
trimethylammonium chloride (AETAC) produced
included: functional groups (FTIR), morphology
(SEM), thermal analysis (DSC).
3 RESULTS AND DISCUSSION
3.1 Isolation of α-cellulose from
Belangke Bamboo
Based on a series of delignification, swelling and
whitening processes that have been carried out in
this study in order to obtain white α-cellulose. At the
isolation stage, α-cellulose is used 75 grams of
belangke bamboo powder and at the end of the
process produces pure α-cellulose of 10.95 grams (as
much as 14.6% of the initial weight of belangke
bamboo used). Figure 1: shows the α-cellulose
results obtained from this study.
Figure 1: α-cellulose powder isolated from bamboo
belangke.
Modification of Pulp Cellulose of Belangke Bamboo (Gigantochloa pruriens) using [2-(Acryloyloxy)Ethyl] Trimethyl Ammonium Chloride
and Maleic Anhydride
307
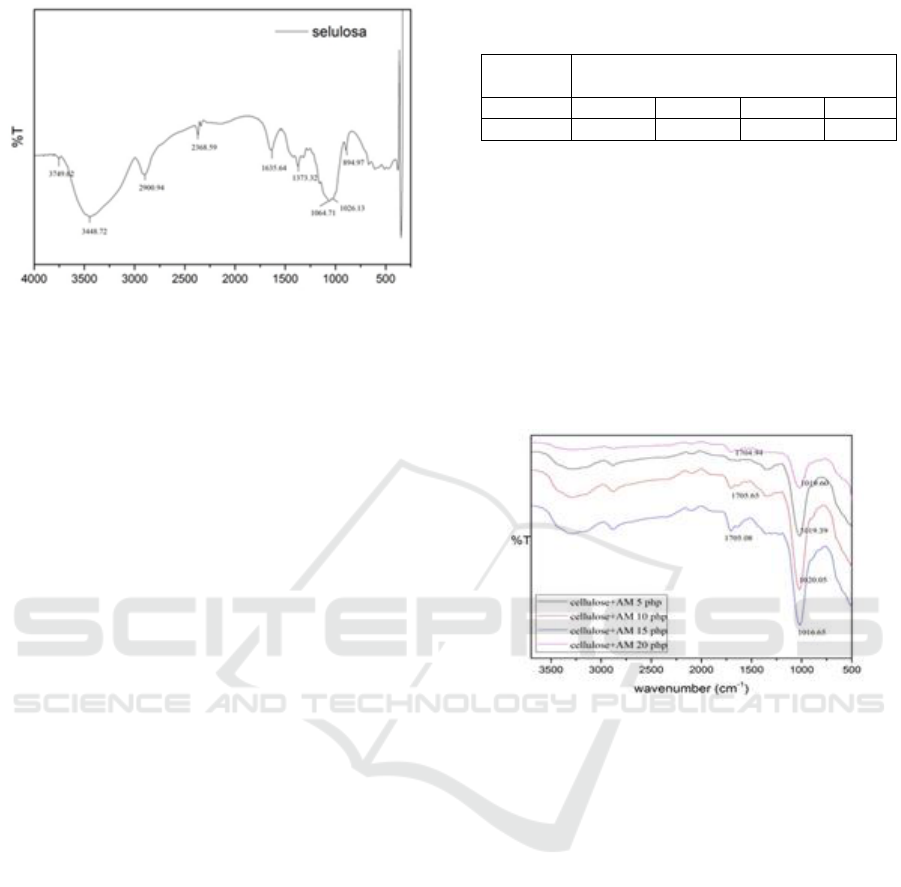
Figure 2: FTIR spectrum of cellulose.
Functional group analysis with FT-IR has been
carried out, for the cellulose spectrum shown from
FT-IR data to provide cellulose support that has an -
OH group with the emergence of vibration peaks at
wave number 3448.72 cm
-1
and supported by peak
absorption at wave numbers 1026.13 cm
-1
which
shows the vibration of the symmetric CO group and
the absorption peak at wave number 1373.32 cm
-1
shows the anti-symmetric CO vibrations. The peak
vibrations at wave number 2900.94 cm
-1
are
stretching C-H vibrations supported by bending C-H
vibrations at wave number 671.23 cm
-1
. The
emergence of the vibration peak at wave number
2368.59 cm
-1
shows the C-C stretching vibration and
is supported by the wave number 894.97 cm
-1
which
shows the C-C bending (Penjumras et al., 2014).
3.2 Modification of Belangke Bamboo
Cellulose α-Maleic Anhydride
In order to optimize the conditions for grafting of
maleic anhydride onto cellulose, we did this by
varying the concentration of monomers. The grafting
mechanism of anhydride groups onto cellulose in the
melt, it has been shown that the grafted polymer
generally contains residual amounts of free
(ungrafted) maleic anhydride as well as free
ungrafted polymaleic anhydride sequences. It has
been shown that the free maleic anhydride can be
removed by vacuum-drying while the free
polymaleic anhydride is removed by washing.
Cellulose grafting with maleic anhydride can be
calculated from the peak FTIR characterization.
Because the absorption coefficient was calculated,
the content of the grafted maleic anhydride onto the
cellulose form can simply be determined by
measuring the FTIR spectra (Krump et al., 2005).
Table 1: Cellulose grafting results with variations in the
concentration of maleic anhydride.
Cellulose
(php)
Maleic anhydride
(php)
100
5
10
15
20
13.6544
0.0269
0.0775
0.1612
0.1131
From the data in Table 1 it appears that the
optimum conditions for the addition of maleic
anhydride at 15 phr. This shows that the increase in
the degree of grafting caused by the cross-ring
formation of polymers and poly (maleic anhydride)
increases. the degree of grafting begins to decrease
when the concentration of maleic anhydride is more
than 15 phr, this is due to the homopolymerization
that causes maleic anhydride monomers tend to form
a polymer themselves compared to sticking to
cellulose.
Figure 3: FTIR spectrum of α-cellulose with various AM
levels.
From the data in Table 2: it can be seen that the
optimum conditions of adding AETAC to 20 phr are
0.6393, when the addition of AETAC 30 phr to the
condition of grafting decreases. This is because the
number of AETAC additions causes the reaction
between maleic anhydride and cellulose not to the
maximum possibility of maleic anhydride monomers
with less colliding cellulose. Grafting is increased if
maleic anhydride monomers with cellulose and
AETAC collide with each other.
Figure 4 shows the results of grafting between
the optimum conditions of AM-cellulose produced
previously with variations in AETAC levels. Of the
three FTIR results obtained, all three AM-cellulose
have been successfully grafted with AETAC. This
can be seen with the emergence of new peaks at
wavelengths of 1463, 1465 and 1467 cm
-1
which are
the tops of the methyl groups of antimicrobial
compounds (AETAC). But after calculating the
surface area by using the previous formula which
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
308
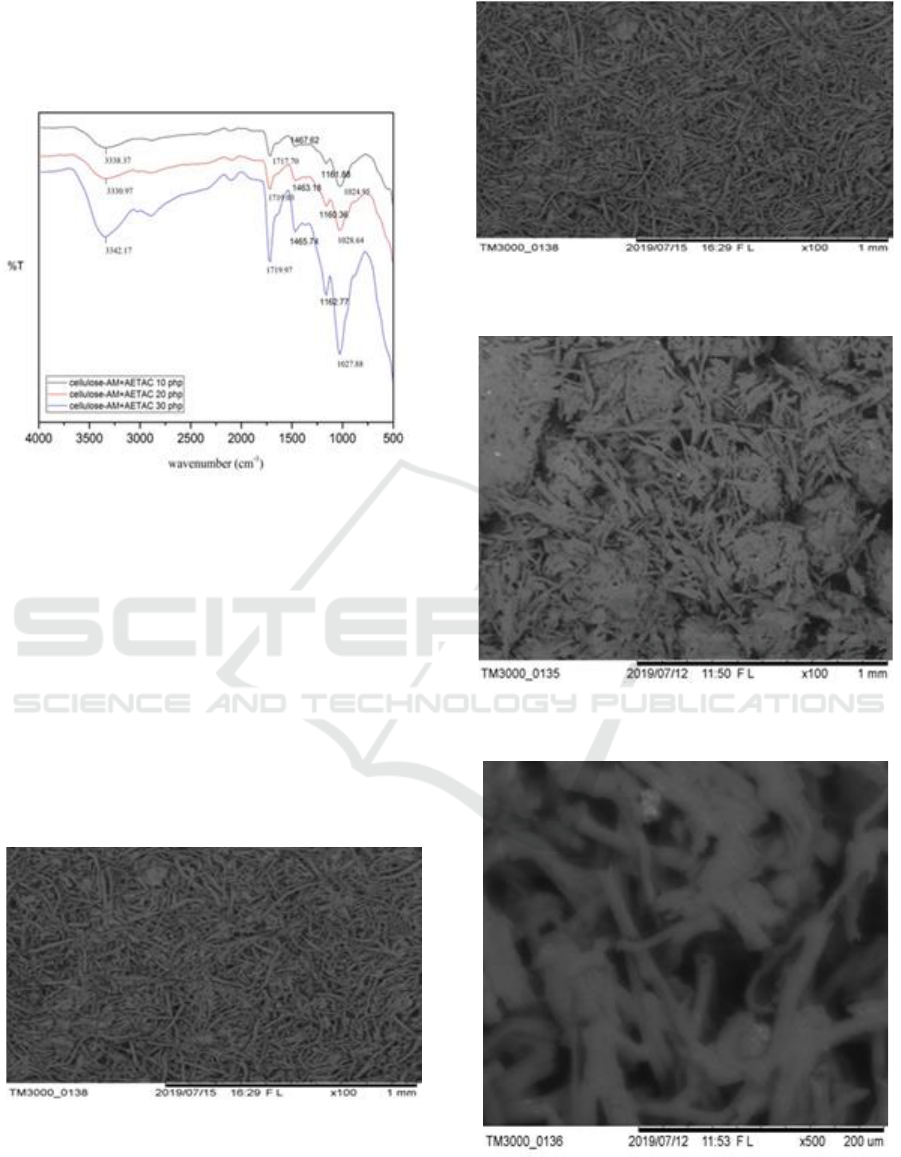
compares the peak of maleic anhydride with one of
the cellulose peaks, it can be produced that the
optimum condition of cellulose-AM-AETAC
modification is on the addition of 20 php AETAC.
Figure 4: FTIR spectrum of α-cellulose-AM with various
AETAC levels.
3.3 Analysis Results Scanning Electron
Microscope (SEM)
The results of the SEM analysis can provide
information about the shape and change of the
material being tested. In principle, if there is a
change in a material such as fractures, indentations,
and structural changes, the material tends to
experience energy changes. The changed energy can
be emitted, reflected, and absorbed and converted
into electron waves that can be captured and read the
results on SEM photographs.
Figure 5: SEM test results of bamboo cellulose surface
with 100x magnification.
Figure 6: SEM test results of bamboo cellulose surface
with 500x magnification.
Figure 7: SEM test results for MA-AETAC cellulose
grafting with 100x magnification.
Figure 8: SEM test results for MA-AETAC cellulose
grafting with 500x magnification.
Modification of Pulp Cellulose of Belangke Bamboo (Gigantochloa pruriens) using [2-(Acryloyloxy)Ethyl] Trimethyl Ammonium Chloride
and Maleic Anhydride
309
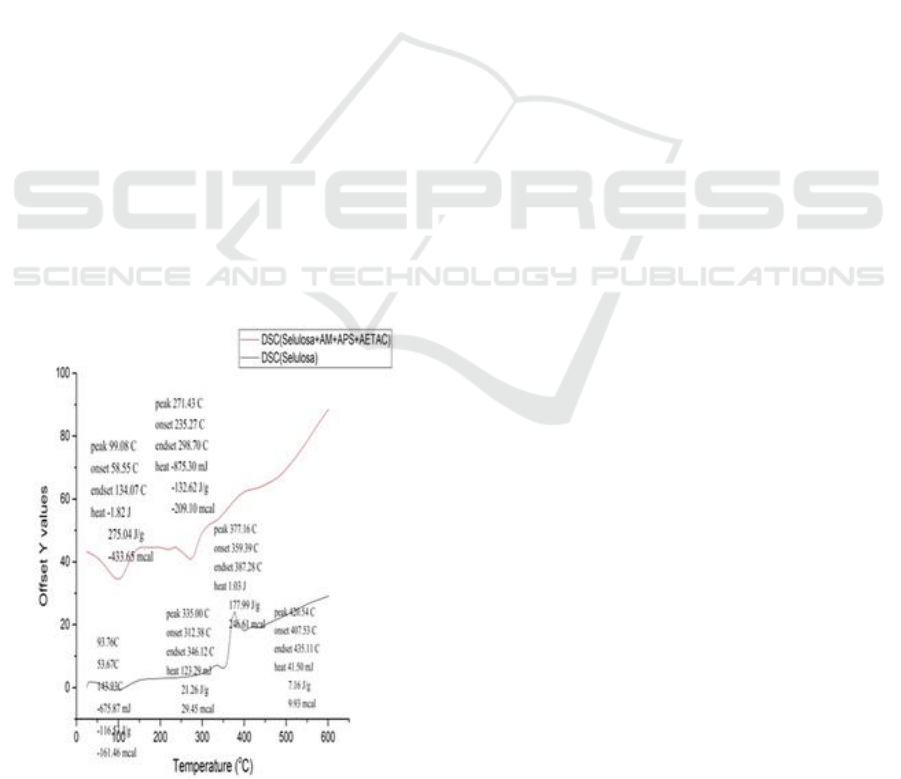
The SEM results shown Figure 7: shows that the
structure is uniform, homogeneous and has small
pores which are cellulose structures from bamboo
belangke. Cellulose has an ion -OH bond that can
cause the adsorption process.
Figure 8: shows that the structure of cellulose has
changed due to the addition of maleic anhydride and
aetac. These changes state that the surface of
cellulose has been grafting maleic anhydride and
aetac, there are some areas where clots occur and
enlarged pores.
3.4 Differential Scanning Calorimetry
(DSC) Analysis
DSC analysis is used to study phase transitions, such
as melting, glass transition temperature (Tg) or
exothermic decomposition and to analyze the
stability of oxidation and heat capacity of a material.
A technique used to determine the temperature of a
material transformation by quantifying its heat. The
data generated in the form of a heat flow curve to the
sample minus the heat flow to the reference to time
or temperature.
In thermal analysis, cellulose decomposes at
temperatures between 270oC to 400oC. In this area,
cellulose decomposes into D-glucopyranose
monomers (Yang, 2008). This can also be seen in
Figure 5: which shows that cellulose is degraded at
400oC and also on the DSC results of cellulose
modification with AM-AETAC degradation at
300oC.
Figure 9: DSC analysis of cellulose and cellulose / AM-
AETAC.
4 CONCLUSIONS
Optimization and reaction mechanism of the
modification process and surface characteristics of
cellulose pulp from Belangke bamboo with the help
of maleic anhydride through FTIR test that is found
in the addition of 15 phr maleic anhydride by
calculating the surface area of FTIR which compares
the peak of maleic anhydride with one of the peaks
of cellulose.
Characterization of functional groups (FTIR),
morphology (SEM) and thermal strength (DSC) of
cellulose, cellulose / AM and AM-AETAC cellulose
grafting namely the emergence of new and
characteristic peaks from cellulose and from maleic
anhydride. The change in cellulose morphology to
cellulose grafting AM-AETAC is marked by a
change in the surface structure in the form of pores,
indentations and faults. In thermal analysis, cellulose
decomposes at temperatures between 270
o
C to
400
o
C. In this area, cellulose decomposes into D-
glucopyranose monomers. This can be seen from the
endothermic and exothermic reactions that occur.
The FTIR results can be calculated the optimum
conditions from the addition of AETAC, namely by
calculating the surface area of the peak maleic
anhydride with one of the cellulose peaks obtained
the optimum conditions for the addition of AETAC
of 20 phr.
ACKNOWLEDGEMENTS
Authors would like to thank to Ministry of RISTEK-
DIKTI, the Republic of Indonesia for financial
support through DRPM program 2018. Furthermore,
additional support from all those who helped the
completion of this research are gratefully
acknowledged.
REFERENCES
Fathanah, U., Lubis, M. R., Othman, N., Handayani, M.
N., & Karlina, S., 2017. Preparation and
Characterization of Cellulose and Nanocellulose from
Agro-industrial Waste - Cassava Peel Preparation and
Characterization of Cellulose and Nanocellulose from
Agro-industrial Waste - Cassava Peel.
https://doi.org/10.1088/1742-6596/755/1/011001
Hassan, M. M., 2015. Binding of a quaternary ammonium
polymer-grafted-chitosan onto a chemically modified
wool fabric surface: Assessment of mechanical,
antibacterial and antifungal properties. RSC Advances,
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
310

5(45), 35497–35505. https://doi.org/10.1039/
c5ra03073k
Krump, H., Alexy, P., & Luyt, A. S., 2005. Preparation of
a maleated Fischer – Tropsch paraffin wax and FTIR
analysis of grafted maleic anhydride, 24, 129–135.
https://doi.org/10.1016/j.polymertesting.2004.09.011
Kumar, G. V., 2011. Studies on Antimicrobial Textile
Finish Using Certain Plant Natural Products,
560059(9980819835).
Mohanty, A., Misra, M., Drzal, L., Selke, S., Harte, B. R.,
& Hinrichsen, G., 2005. Natural fibers, biopolymers,
and biocomposites: An introduction. In Natural Fibers,
Biopolymers, and Biocomposites (pp. 1–36).
My, T., & Le, A., 2015. Overview of bamboo biomass for
energy production to cite this version: 0–24.
Ohwoavworhua, F. O., & Adelakun, T. A., 2005.
Phosphoric Acid-Mediated Depolymerization and
Decrystallization of α - Cellulose Obtained from Corn
Cob: Preparation of Low Crystallinity Cellulose and
Some Physicochemical Properties, 4(December), 509–
516.
Penjumras, P., Abdul, R. B., Talib, R. A., & Abdan, K.,
2014. Extraction and Characterization of Cellulose
from Durian Rind. Italian Oral Surgery, 2, 237–243.
https://doi.org/10.1016/j.aaspro.2014.11.034
Shen, S., Zhu, Z., & Liu, F., 2016. Introduction of poly
[(2-acryloyloxyethyl trimethyl ammonium chloride) -
co - (acrylic acid)] branches onto starch for cotton
warp sizing. Carbohydrate Polymers, 138, 280–289.
https://doi.org/10.1016/j.carbpol.2015.11.058
Sun, J. X., Sun, X. F., Zhao, H., & Sun, R. C.,2004.
Isolation and characterization of cellulose from
sugarcane bagasse. Polymer Degradation and Stability,
84, 331–339. https://doi.org/10.1016/j.
polymdegradstab.2004.02.008
Tian, C., 2013. Improvement in the Fock test for
determining the reactivity of dissolving pulp. Tappi
Journal (Vol. 12).
Waite, M., 2009. Sustainable Textiles: The Role of
Bamboo and a Comparison of Bamboo Textile
Properties, 6(2), 1–21.
Yang, Z., Xu, S., Ma, X., & Wang, S., 2008.
Characterization and acetylation behavior of bamboo
pulp. Wood Science and Technology (Vol. 42).
https://doi.org/10.1007/s00226-008-0194-5
Zakikhani, P., Zahari, R., Sultan, M. T. H., & Majid, D.
L., 2016. com Thermal Degradation of Four Bamboo
Species, 11(Yu 2007), 414–425.
Modification of Pulp Cellulose of Belangke Bamboo (Gigantochloa pruriens) using [2-(Acryloyloxy)Ethyl] Trimethyl Ammonium Chloride
and Maleic Anhydride
311
