
Preparation and Characteristics of Polyvinyl Alcohol-based Hydroel
Containing Natural Microbentonite
Mawarani Manullang
1
, Diana Adnanda Nasution
2
and Basuki Wirjosentono
2*
1
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Jl. Bioteknologi
No. 1, Medan 20155, Indonesia
Keywords: Polyvinylalcohol, Natural Microbentonite, Acrylicacid, Interpenetrating-hydrogels.
Abstract: In this work, polyvinylalcohol (PVA)-based interpenetrating-hydrogels containing natural microbentonite
(NMB) were prepared in a bench scale reflux-reactor using water as solvent in an optimized condition in the
presence of constant compositions of acrylicacid (AA) and N,N’-methylenebisacryamide (MBA) as
comono-mers as well as ammoniumpersulphate [(NH
4
)
2
S
2
O
8
, APS] as initiator. Results of interpenetrating-
hydrogels, with various loadings of NMB (2, 5, 10 and 15%) were moulded and cooled to form film
specimens, and then characterized for their properties. First of all, their mechanical properties and chemical
structure were measured using tensile testing (ASTM D882) and infrared spectroscopy (FTIR), then their
thermal properties were analysed using differential scanning calorimetry (DSC), and their morphological
properties were tested using scanning electron microscopy (SEM). Results showed that optimum
composition weight ratio of PVA/AA/NMB/MBA/APS = 60/40/10/1/0.5 enhanced highest tensile modulus
of the interpenetrating-hydrogel microcomposites 25% in compare to its neat hydrogel counterpart. FTIR
analysis of the film speci-mens after exhaustive extraction in n-hexane still contained stable AA-carbonyl
(C=O) peak at 1725 cm
-1
. Thermal properties of the optimized composition of the hydrogel showed slight
improved of thermal stability (decomposition temperature increase from 280 – 285
o
C). Morphological
properties of the interpenetrating-hydrogel microcomposite also showed finely distributed of the microfiller,
which is responsible for its improved mechanical and thermal properties. It is recommended that the
interpenetrating-hydrogels can be further developed for application as biomedical materials.
1 INTRODUCTION
Along with the increasing industry in Indonesia, all
the raw material needs derived from rock materials
also increase, one of which is bentonite. Bentonite is
a type of clay whose availability is very abundant in
Indonesia. Indonesia has a source of local raw ma-
terials based on natural polymer hydrogels, namely
bentonite. The potential of bentonite in Indonesia is
quite large and spread in several locations, namely
the island of Java and Sumatra. one of them in the
Province of Nanggroe Aceh Darussalam which is
located on the western tip of the island of Sumatra.
In this area the source of bentonite is still not widely
processed by the government and industry. Benton-
ite used as filler was first made into bentonite nano-
particles using the coprecipitation method. Hydrogel
is a hydrophilic polymer network that is cross-linked
and has the capacity to expand (swelling) by ab-
sorbing water or biological fluids but is insoluble
due to crosslinking. Hydrogel from poly (acrylic
acid) -ko-polyvinyl alcohol can be synthesized from
cranic acid and polyvinyl alcohol using gamma
irradiation (Nesa, 2017). This is indicated by an
increase in absorption (Erizal, 1998). The ability of a
hydrogel to absorb water that is insoluble in water is
indicated by the presence of cross connective tissue
when ex-posed to water will form a three-
dimensional mac-romolecular network (Zohuriaan-
Mehr, 2008).
Cross-linking in bentonite can improve the char-
acteristics of bentonites such as solubility in water,
or organic solvents, bacteriostatic effects, chelating
ability and complexing. Processing with N, N
dengan-methylene bisacrylamide (MBA) acrylic
acid as a crosslinker is expected to bind natural
Manullang, M., Nasution, D. and Wirjosentono, B.
Preparation and Characteristics of Polyvinyl Alcohol-based Hydroel Containing Natural Microbentonite.
DOI: 10.5220/0008928103010304
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 301-304
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
301

nanobentonite added in the interpenetration net-work
of the formed polyvinylalkyton-polyacrylate
hydrogel polymer. Polyvinylalkohol (PVA) is an
environmentally friendly polymer that is widely
used as a medical polymer, including: soft lenses,
absorbents, wound dressings, drug carriers, cosmet-
ics, and so on. PVA applications as absorbents are
hampered by their low mechanical properties so they
are easily destroyed, especially if applied in
excessive water medium (Kobayashi et al.,2008).
2 MATERIALS AND METHODS
2.1 Materials
Bentonite. Other reagents are commercially
available. Acrylic acid (AA) and Ammonium
Persulfate (APS) are used as initiators of
polymerization, Poly-vinylalkohol (PVA), N, N'-
methylenbisacrylamide (MBA).
2.2 Preparation PVA 10%
Technical PVA is weighed as much as 10 grams,
then dissolved into 100 mL of distilled water, steri-
lized and heated at 90°C for ± 3 hours until dis-
solved.
2.3 Making Bionanocomposite
Hydro-gel Interpenetration
The reflux device is equipped with a 250 mL 4 neck
flask, mechanical stirrer, condenser and
thermometer. Entered 6 mL aquadest, added
nanobentonite with a variation of weight 0; 0.2; 0.4;
0.6 and 0.8 and heated the mixture at 60
o
C with a
water bath and stirred constantly until it turns into a
sticky solution like a transparent paste. Enter 10%
PVA solution with variations of 1 ml, 2 ml, 4 ml, 6
ml and 8 ml into the reaction flask. Put 7 ml AA
with 0.04 g N, N'-metilenbisakrilamida (MBA) into
the reaction flask. After bubbling, nitrogen gas is
poured for 30 minutes. 0.05 g of Ammonium
Persulfat (APS) was added into the reaction flask
with a mechanical stirrer. The reaction mixture
temperature is raised to 60
o
C and the reaction is
maintained for 1 hour. Soaked with ethanol products
Which is produced for 24 hours. Dried in the oven
for 24 hours at 60
o
C.
2.4 Characterization
2.4.1 Analysis of FTIR
FTIR analysis was used to determine the functional
groups found in graphene oxide. Then the sample is
prepared in liquid form (suspension), then the sam-
ple is dropped between two KBr plates to make a
thin film. Then the plate is placed on the sample to
be passed by infrared light. FTIR analysis using the
Prestige-21 IR device was carried out in the wave
number range 4500-450 cm
-1
.
2.4.2 Morphological Analysis with SEM
The microscopic observation process using SEM
was carried out on the sample fault surface. Then
after the sample is cleaned with a blower, the sample
is coated with gold and palladium in a machine that
presses 1492 x 10
-2
atm, the sample is then put into a
room (vacuum evaporator) with a pressure of 0.2
Torr using the JSM-35 C Shimadzu machine .
Furthermore, the sample is irradiated with a 20 kV
electron beam in a special room so that the sample
emits secondary electrons and electrons which are
bounced can be detected by the Scientor detector
which is amplified by an electrical circuit that causes
a 4 minute Cathode Ray Tube (CRT) to appear.
Then the 400 Armstrong coating is inserted into the
Chamber specimen to be carried out by removing
secondary electrons and the detectors can be
detected by the detector. The shooting results can be
adjusted to the desired magnification for shooting.
2.4.3 Test the Percentage of Water
Absorption
Testing the percentage of water absorption was car-
ried out by determining the percent swelling ratio by
measuring the initial weight (Wd) of the sample
which was then immersed in distilled water for 24
hours. use filter paper and measure the final weight
again (Ws). Measuring the percentage of water
absorption in the hydrogel can be determined by the
following formula:
Where:
Degree of cross tie (%) =
𝑊𝑎
Wb
𝑥 100% (1)
Wa = weight of dry hydrogel after soaking
Wb = weight of dry hydrogel before immersion.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
302
3 RESULTS AND DISCUSSION
FTIR functional group test results on bentonite hy-
drogel, 2 ml PVA: 7 ml AA, and APS 0.05: NBA
0.2 grams with the addition of MBA as much as 0.04
grams can be seen in Figure 1. Hydrogel that has
been made in cluster analysis its function using
FTIR. The main functional group of bentonite con-
stituents can be seen through absorption of the OH
group at 2985.16 cm
-1
, Si-OH-Si group at 1027.67
cm
-1
, AL-O-Si group at 799.30 cm
-1
, Si-O group at
521.99 cm
-1
, the 0-H group bending at 978.74 cm
-1
and at 3392.96 cm
-1
and 1647.54 cm
-1
are adopted
water molecules. The above functional group is the
main functional group of bentonite in the hydrogel.
This result is in accordance with the research
(Darvishi, 2010).
Figure 1: FTIR of hydrogel bentonite 0,2 g.
Figure 2 shows the results of SEM 500x
magnification in PVA / AA / APS / NBA / MBA
hydrogel composite samples. Because PVA
dissolves in water (Gao, 2015). whereas bentonite is
a hydrophilic polymer and swelling in water, the
three can be mixed like shown in Figure 2. In
general, it can be seen that the morphology of the
full PVA / AA / APS / bentonite / MBA hydrogel
SEM has a rough surface and large density. This
shows that the polymer network formed will
converge and interact with each other. Irregular
shapes provide porous space so that absorption can
occur. Heterogeneous surfaces indicate that there are
areas that experience interactions between bentonite
chains and there are also areas that do not form
interactions of the bentonite chain.
Figure 2: SEM image of NBA 0,4 g.
Figure 3: Graph of Absorption Test Results.
Based on the table and graph above, shows that
the hydrogel semi-interpenetration polymer network
on the weight of the addition of bentonite is 0.4 g;
0.6 g and 0.8 g still have low absorption compared
to the blank. It can be said that the more the amount
of bentonite used, the smaller the value of the
absorbed water. The solvent re-sistance test is
carried out to see how far the hydrogel is resistant to
solvents such as water. The polymer Material for the
build up of the hydrogel should inflate (swell) and
retain the water fraction of its structure, but not
soluble in water. Both natural and synthetic
materials have been widely used to synthesize
hydrogels (Dragan et al., 2012)., (Matricardi,2013).
Figure 4: Graph of Absorption Test Results.
Preparation and Characteristics of Polyvinyl Alcohol-based Hydroel Containing Natural Microbentonite
303
Hydrogels with crosslinked structures can absorb
water, but are not soluble in water. The process of
entering water into the hydrogel matrix results in the
development of the hydrogel it self (Yang,2012).
Revealed that at the beginning of the absorption
process, water diffuses in the network continuously
until it reaches equilibrium conditions, with
maximum development. After the equilibrium phase,
the amount of water absorbed decreases due to the
gel liquefaction cycle. In Figure 4, the percent of
devel-opment increases as a function of time and
reaches the maximum condition after ± 24 hours of
immer-sion, for all bentonite / PVA hydrogel
concentrations which are 0 g; 0.2 g; 0.4 g; 0.6 g and
0.8 g.
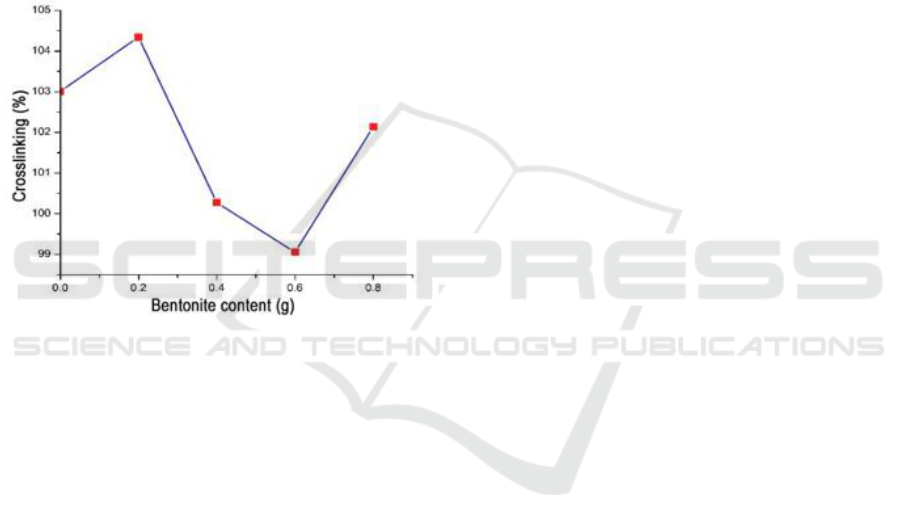
Figure 5: Graph of test results% Crossed.
4 CONCLUSIONS
Optimum condition obtained through hydrogel
absorbent power test and superabsorbent cross tie
test using full IPN method with a variation of time 5
minutes, 10 minutes, 20 minutes, 30 minutes, 90
minutes 1 day and 2 days with variations of ben-
tonite 0.2 grams. Cross-binding agent concentration
of MBA 0.4%, and the concentration of APS
initiator 0.5%. The value of this site hydrogel ratio is
533.83% and the cross belt is 104.34%. The power
of the bentonite structure can be improved by
conducting polymerization using the full IPN
method.
ACKNOWLEDGEMENTS
This research was funded by the DRPM Republic of
Indonesia Ministry of Research and Technology
Republic of Indonesia Fiscal Year 2019.
REFERENCES
Nesa. F., 2017. Sintesis dan pencirian hidrogel poli (asam
akrilat)-ko-polivinil alkohol dengan teknik iradiasi
gamma. Skripsi Departemen kimia. FMIPA IPB.
Erizal,, and Rahayu, C,.1998. Characterization of poly
(vinyl alcohol) (PVA) hydrogel prepared by radia-tion
polymerization; Karakterisasi hidrogel PVA hasil
polimerisasi radiasi. Indonesia
Zohuriaan-Mehr, M. J., & Kabiri, K., 2008. Superabsor-
bent polymer materials: a review. Iranian polymer
journal, 17(6), 451.
Kobayashi M., and S. H. Hyon., 2010, Development and
Evaluation of Polyvinyl Alcohol-Hydrogels as an Arti-
ficial Atrticular Cartilage for Orthopedic Implants,
Materials, 3, 2753-2771.
Darvishi, Z., 2010. Morzali, A. Ultrason. Sonochem. 18,
238–242
Gao,Q., He,Y., Fu,J., Liu,A., and MaA.,2015. “Coaxial
nozzle-assisted 3D bioprinting with built-in micro-
channels for nutrients delivery,” Biomaterials, vol. 61,
pp. 203–215.
Dragan, E. S.;Perju, M. M.;& Dinu, M. V., 2012. Prepa-
ration and characterization of IPN composite hydrogel
based on polyacylamide and chitosan and their inter-
action with ionic dyes. Carbohydrate Polymers, 270-
281
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
304
