
Synthesis 2-(3-Phenylallylidenamino) Pentanedioic Acid by
Condensation of Cinnamaldehyde with Glutamic Acid
and the Activity Test as Antibacterial
Mimpin Ginting*, Deni Aprilina Lumban Gaul, Indra Masmur and Andy Candra
Departement of Chemistry,Faculty Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan
Keywords: Antibacterial, Condensation, Cinnamaldehyde, Glutamic Acid, Schiff Base.
Abstract: 2-(3- Phenylallyldenamino) Pentanedioic Acid as Schiff base has been synthesize by condensation between
cinnamaldehyde and glutamic acid as the source of primary amine. Condensation between cinnamaldehyde
and glutamic acid is in reflux condition by using ethanol as a solvent for 5 hours. The yield’s percentage of
reaction is 49.39%. The formed Schiff base is proven by FT-IR spectrum with the appearance of a vibration
peak at wave number in 1627 cm
-1
as the sign of C=N functional group. UV-Vis spectrum showed the change
of cinnamaldehyde maximum wave number in 238 nm become Schiff base in 321 nm. The result of research
also show that the Schiff base has strong antibacterial activity for S. aureus by obstacle zone 13.3 mm and
weak antibacterial activity for E. coli by obstacle zone 6.6 mm.
1 INTRODUCTION
Condensation between primary amine with
carbonyl from ketone and aldehyde in organic
compounds in special condition will produce Schiff
base (Cimerman et al. 1997, da Silva, et al, 2011).
Schiff base is known structurally as azometine (-
CH=N-) and one of organic compound with many
uses like as pigment and dye, catalyst, intermediary
in organic synthesize, and polymer stabilizer (Dhar
and Taploo, 1982). Schiff base also has biology
activity such as antifungal, antibacterial, antimalarial,
antiproliferative, anti-inflammation, antivirus, and
antipyretic (Dhar and Taploo, 1982, Przybylski, et al,
2009). The different use of Schiff base is based on
basic material from aliphatic, aromatic, heterocyclic
of primary amine and the source of carbonyl in use
(da Siva, et al, 2011).
Many researches also prove that Schiff base is
very effective as corrosion inhibitor for metal by form
a layer to protect material environmentally (Li et al.
1999, Munir, at al, 1985). The former researcher also
tested Schiff base from cinnamaldehyde, 2-
aminophenol condensation as corrosion inhibitor for
iron in HCl 0.5 N with inhibitor efficiency 92%
(Qasim, 2011). Schiff base from cinnamaldehyde as
the source of carbonyl and ethylenediamine as the
source of amine also can be used as corrosion
inhibitor in 7000 ppm of zinc in HCl 0.1 N with
efficiency 90.17% (Ginting, et al, 2016). Beside as
corrosion inhibitor, Schiff base also has antimicrobial
properties (Amanullah et al, 2011). Aysen et al (2010)
also synthesize Schiff base by using 4-
benzylloxybenzaldehide with 2-aminophenol and the
result has good antibacterial properties in E. coli,
B.subtilis, S. aureus (Sirumapea et al, 2015). Wang
et al (2015), synthesize Schiff base from
cinnamaldehyde with some of amino acid and found
that it has antibacterial properties in Eschericia coli,
Aspergillus niger, Penicillium citrinum and
Staphylococcus aureus. Cinnamaldehyde is a natural
product kind of phenylpropionate (C6-C3) which is
instead of synthesizing, it is also as the main
component of cinnamon oil (Guenther, 1990). The
utilization of Schiff base should be improved by
changing cinnamaldehyde to its derivative. By its
chemical properties, cinnamaldehyde has benzene
ring, alkene, and aldehyde so it can be transformed to
cinnamaldehyde derivatives (Ngawidiyana et al,
2007). Glutamic acid is an amino acid used by
organism in protein biosynthesis. This kind amino
acid is one of nonessential amino acid for human
which mean it can be synthesized in their body
(IUPAC-IUB, 2008). Glutamic acid as component of
protein contain in food, but only can be tasted in
Ginting, M., Aprilina Lumban Gaul, D., Masmur, I. and Candra, A.
Synthesis 2-(3-Phenylallylidenamino) Pentanedioic Acid by Condensation of Cinnamaldehyde with Glutamic Acid and the Activity Test as Antibact.
DOI: 10.5220/0008919702290233
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 229-233
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
229

original form. Most of glutamic acid is exist in many
foods like cheese and ketchup. Glutamic acid also
used as additive in food, and as flavor in its natrium
salt known as monosodium glutamic (MSG). Based
on the description, the researcher interest to
synthesize Schiff base by using cinnamaldehyde with
glutamic acid and it used as antibacterial.
2 MATERIALS AND METHODS
2.1 Materials and Equipment
The tools used in this study include: two neck flasks,
reflux devices, thermometers, glassware, vacuum
pumps, analytical balance sheets, chromatographic
columns, chambers, UV lamps, petri dishes, osseous
needles, paper discs, incubators, FT-IR
spectrophotometer, UV-Vis spectrophotometer.
While the materials used in this study include:
Cinnamaldehyde, ethanol, glutamic acid, ethyl
acetate, n-hexane, silica gel HF254, silica gel 60 are
all pro-analysis made by E. Merck.
2.2 Synthesis of 2- (3- Phenyl
alylidinamine) Pentanadioate Acids
About 6.6 g of cinnamaldehyde were dissolved in 25
mL of ethanol, then put in a two-volume 250 mL neck
flask. Next, a drop of 5.9 g of glutamic acid was
dissolved with 50 mL ethanol through a dropper
funnel slowly into the mixture. Then reflux for 5
hours while stirring. Furthermore, the ethanol solvent
used was evaporated with rotary evaporator. Excess
Cinamaldehyde is evaporated by vacuum distillation
at 100oC and a pressure of 20mmHg. The residual
weight was obtained, then thin layer chromatography
(TLC) analysis was performed using developer n-
hexane: ethyl acetate (8: 2 V / V), kiesel 60 HF254
adsorbent and UV lamp stain, then purified using
column chromatography. The results obtained were
analyzed by FT-IR and UV-Vis spectroscopy
followed by an antibacterial activity test.
2.3 Schiff Base Analysis Synthesis
The purity of the Schiff base was analyzed using thin
layer chromatography, using several developers and
mixed developers with the stationary phase of silica
gel HF254. The analysis results provide a single stain.
The results obtained were then carried out FTIR
spectroscopic analysis with KBr and UV-Vis pellet
media in ethanol solvents then followed by an
antibacterial activity test.
2.4 Antibacterial Activity Test
2.4.1 Making Nutrient Agar Media
A total of 7 g was dissolved with 250 ml of aquadest
in an Erlenmeyer glass and heated to dissolve and
boil, then sterilized in an autoclave at a temperature
of 121
o
C for 15 minutes.
2.4.2 Manufacture of Oblique and Stock
Media for Bacterial Culture
In a sterile test tube, 3 ml of sterile NA media are
inserted, left at room temperature until it solidifies at
an angle to form an angle of 30-45
o
C. Bacterial
culture from the main strain was taken with a sterile
osseous needle and then inoculated on the sloping NA
media surface by scraping, then incubated at 35
o
C for
18-24 hours.
2.4.3 Making Mueller Hinton Agar Media
(MHA)
As much as 19 g of Mueller Hinton Agar powder, put
in an Erlenmeyer glass and then dissolved in 500 ml
of aquadest and heated until all dissolve and boil.
Then sterilized in an autoclave at 121
o
C for 15
minutes.
2.4.4 Making Bacterium Inoculum
A total of 3.25 g of nutrient broth was dissolved in
250 ml of aquadest in an Erlenmeyer glass and heated
to all dissolve and boil, then sterilized in an autoclave
at 121
o
C for 15 minutes and cooled. Then the
microbial colonies were taken from the stock of
culture using sterile osseous needles then suspended
into 10 ml sterile nutrient media in a test tube and
incubated at 35
o
C for 3 hours, then measured the
wavelength using a UV-Vis spectrophotometer
wavelength 580-600 nm.
2.4.5 Determination of Antibacterial
Activity
Determination of antibacterial activity was obtained
by agar diffusion method where the paper disk (ǿ 6
mm) which had been immersed with Schiff Bases 3%
and in direct contact with the media which had been
inoculated by E. coli and S. aureus, then clear zones
formed after incubation were observed for 24 hours.
The clear zone shows the inhibition that is produced
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
230
from the Schiff Base on E. coli and S. aureus. The
clear zone formed is measured by using the calipers
(accuracy mm).
3 RESULT AND DISCUSSION
3.1 Synthesis of Bases Schiff
The Schiff base is a 2- (3- Phenylalylidinamine)
Pentanadioate which is produced through
condensation between cinnamaldehyde as a source of
aldehyde and glutamic acid as a source of amine in
ethanol solvents under reflux conditions for 5 hours.
The excess of cinnamaldehyde from the reaction
results was evaporated through vacuum distillation
and analyzed by thin layer chromatography using
developer n-hexane: ethyl acetate (8: 2 v / v), the price
of product Rf still mixed was 0.11 and 0.75. From 6.6
g of cinnamaldehyde used the results were as much as
9.47 g (93.20%) yellowish brown solids. The
purification is continued through column
chromatography with eluent n-hexane: ethyl acetate
(8: 2 v / v), where purification results are 0.53 g, so
that the total yield is 49.39%. The results obtained
were analyzed using thin layer chromatography using
developer n-hexane: ethyl acetate (8: 2 v / v) giving a
single stain at an Rf price of 0.67. The physical form
of the Schiff base obtained is a solid form with a
melting point 124-129
o
C indicating that changes have
been made from the solid base material namely
glutamic acid with a melting point 247- 249
o
C. The
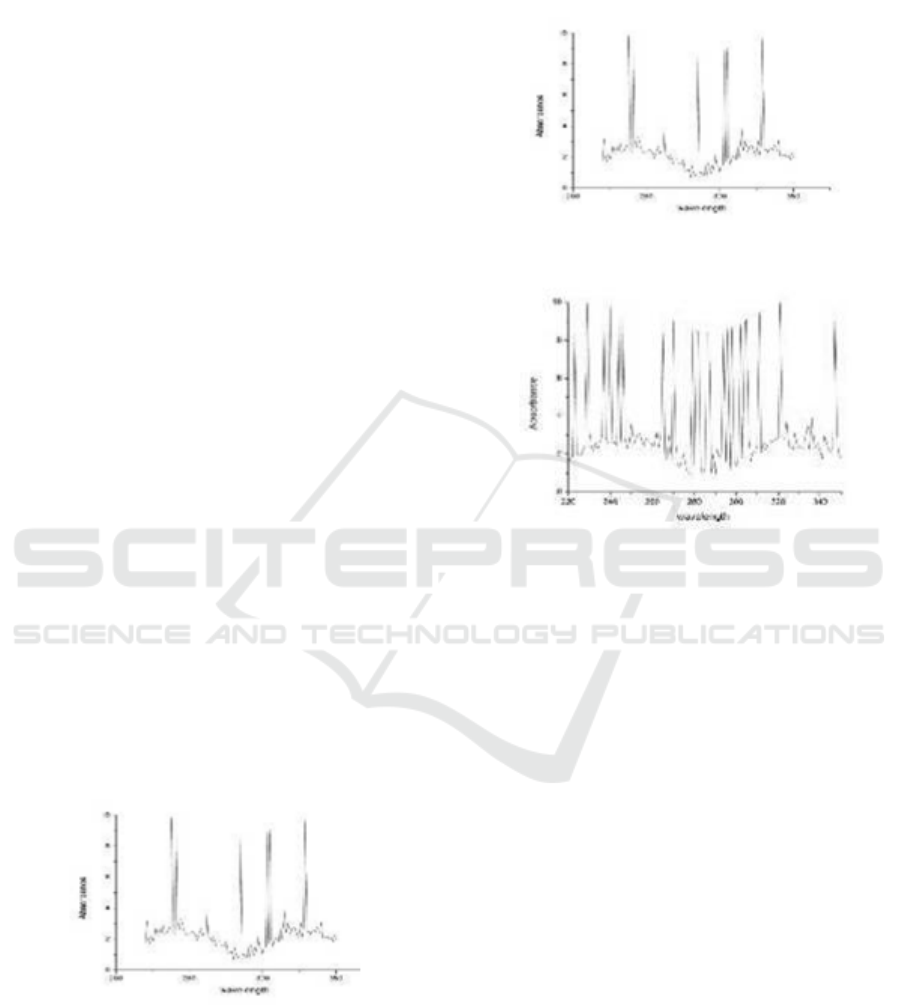
results of the Schiff base analysis produced using FT-
IR spectroscopy obtained spectra with absorption
vibration peaks at the wave number area 3410 cm
-1
,
3024 cm
-1
, 2924 cm
-1
, 1720 cm
-1
, 1674 cm
-1
, 1627 cm
-1
, 1450 cm
-1
, 1126 cm
-1
(Figure 1).
Figure 1: FT-IR Spectrum of Schiff Base.
From the results of UV-Vis spectroscopy there was
an increase in wavelength of 238 nm for
cinnamaldehyde (Figure 2) to 321 nm for the Schiff
base (Figure 3). This shows the addition of
conjugated double bonds to the products produced
due to the presence of new functional groups that are
bound to the synthesized part of the molecule.
Figure 2: UV-Vis Spectrum of Cinnamaldehyde.
Figure 3: UV-Vis Spectrum of Schiff Base.
The Schiff base results from a condensation reaction
between cinnamaldehyde and glutamic acid as
evidenced by the support of the FT-IR spectrum
which shows the emergence of a stretch of C = N at
the absorption peak of the wave number 1627 cm
-1
supported by stretching C-N at wave number 1126
cm
-1
. Uptake in the wave number area 3024 cm
-1
shows that C-H aromatic from the benzene ring
supported by stretching absorption of 1674 cm
-1
shows the vibration of C = C of aromatic compounds.
This is also supported by the vibration peak in the
wave number region 2924 cm
-1
which shows the
typical absorption of vibration stretching (C-H) sp
3
which is supported by bending vibration (C-H) sp
3
in
the wave number region of 1450 cm
-1
. Hypothetically
the Schiff base formation reaction is shown in Figure
4.
Synthesis 2-(3-Phenylallylidenamino) Pentanedioic Acid by Condensation of Cinnamaldehyde with Glutamic Acid and the Activity Test as
Antibact
231
Figure 4: Schiff Base Formation Reaction.
3.2 Antibacterial Activity Test
Antibacterial tests on the Schiff base using
Escherichia coli and Staphylococcus aureus can be
seen in Table 1.
Table 1: Antibacterial Activity Test.
Bacteria
Inhibited Zone
Diameter (mm)
Diameter Disc
Paper (mm)
E. coli
6.6
6.0
S. aureus
13.3
6.0
Cluster C = N in the Schiff base can be antibacterial
where the nitrogen atom has free electrons. The
presence of an imine group which has a cationic
charge that is able to bind the food source of the
bacterium thus inhibits food nutrition into bacterial
cells (Darmanto et al., 2010). Antibacterial strength is
classified into 3, which is strong if it produces a
diameter inhibition zone of more than 8 mm,
moderate activity if it produces a 7-8 mm inhibition
zone diameter, and weak activity if it has a diameter
inhibition diameter of less than 7 mm, thus that the
Schiff base is formed has strong antibacterial
strength. The wider the inhibition zone produced
shows the stronger Schiff's base ability in inhibiting
bacterial growth. The antibacterial test data showed
that the Schiff base had good antimicrobial activity
against the Staphylococcus aureus bacteria which is a
common bacterium outside the body. For Escherichia
coli bacteria, the Schiff base is not very good.
Escherichia coli bacteria are commonly found in the
body.
4 CONCLUSIONS
The condensation reaction between 6.6 g of
cinnamaldehyde and 5.9 g of glutamic acid produced
a Schiff base of 9.47 g obtained for a total yield of
49.39%. Supported by FT-IR spectroscopic data,
namely by the appearance of a vibration peak in the
area of the wave number 1627 cm
-1
which indicates
the group C = N (Imina). The UV-Vis data showed a
change in the wavelength of 238 nm for
cinnamaldehyde to 321 nm for the Schiff base. The
results of the Schiff alkaline antibacterial activity test
showed strong results against the Staphylococcus
aureus bacteria and were weak for Escherichia coli
bacterial.
REFERENCES
Amanullah M, Sadozail SK, Rehman W, Hasan Z,
Rauf A, Iqbal M, 2011. Cytotoxic, Antibacterial
activity and Physico- chemical properties of some
acid catalyzed Schiff bases. African Journal of
Biotechnology, 10(2): 209213
Aysen DM, Birol O, Bedrettin M. Synthesis and
Antibacterials Activity Of Schiff Base Derivate,
2010. International Journal of Drug Development
and Research. 2(1): 102-107
Cimerman Z, Galic N, and Bosner B, 1997. Anal.
Chim. Acta. 343(1997) 145
Darmanto M, Atmaja L, And Nadjib M, 2010. Studi
Analisis Antibakteri dari film Gelatin- kitosan
menggunakan Staphylococus aureus. Prosiding
Skripsi Semester Genap 2010/2011. Surabaya.
Jurusan Kimia. FMIPA. Institut Sepuluh
November
da Silva,C.M, da Silva, D.L, Modolo, L.V,
Alves,R.B, de Resende,M.A, Martin, C.V.B, de
Fatima, A, 2011, Shiff bases: A Short review of
their antimicrobial activities, Journal of Advanced
Research (2), 1-8.
Dhar, D.N, Thappo, C.L, 1982, Schiff Bases and their
Aplications, J,Sci. Ind. Res, 41(8),501-506
Ginting, M, Sihotang, H., Manalu, R, 2016, Sintesis
Basa Schiff Hasil Kondensasi Sinamaldehida
Dengan Etilendiamina dan Fenilhidrazin Serta
Pemanfaatannya Sebagai Inhibitor Korosi Pada
Logam Seng, Prosiding Semirata Bidang MIPA
2016, BKS-PTS Barat, Palembang, 1922-1928.
Guenther, E, 1990. The Essential Oil. Jilid II. Jakarta.
UI-Press
IUPAC-UIB, 2008. Nomenclature and Symbolism
Foramino Acid and Peptides. Joint Commission
on Biochemical Nomenclature
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
232

Li S, Chen S, Ma H, Yu R and Liu D, 1999. Corrosion
Sciences. 41.1273
Munir C, Yousaf SM and Ahmad N, 1985. J. Chem
Soc. Pak, 7(4),301. Dalam Qasim, M. 2011.
Synthesis and Characterization of new Schiff
bases and evaluation as Corrosion Inhibitor. Iraq.
Departement of Chemistry University of Basrah.
Ngadawiyana, Ismiyarto dan Purwantodiningrum,
20017, Reduksi Sinamaldoksin menggunakan
system Katalis Zn/NH4Cl, Semarang, FMIPA
UDIP, Semarang.
Przybylski,P, Huczynski, A, Pyta, K, Breezinski B,
Barti, F, 2009, Biologil Propertis Of Schiff Bases
and azo Derivatives Of Phenols, Curr Org Chem,
13(2), 124-128
Qasim M, 2011. Synthesis and characterization of
new Schiff bases and evaluation as Corrosion
Inhibitor. Iraq: Departement of Chemistry
University of Basrah
Sirumapea LA dan Khoirunisa A, 2015. Sintesis dan
Karakterisasi Senyawa Antibakteri Kompleks Fe
(III) dengan
Derivat Schiff Base. Sumedang. Fakultas Farmasi.
Universitas Padjajaran
Wang H, Yuan H, Li S, Li Z, Jiang M, 2015.
Synthesis, Antimicrobial Activity of Schiff base
Compounds of Cinnamaldehyde and Amino
Acids. Bioorganic and Medicinal Chemistry
Letter. China: Northeast Forestry University
Synthesis 2-(3-Phenylallylidenamino) Pentanedioic Acid by Condensation of Cinnamaldehyde with Glutamic Acid and the Activity Test as
Antibact
233
