
Production of Bioethanol Gel from Sugar Cane Waste with Carbopol
as Alternative Fuel
Wilza Fithri Az-zahra, N. Nurlina Harahap, S. Haidar Putra and M. Zulham Efendi Sinaga*
Chemistry Department, Faculty of Mathematic and Natural Science, Universitas Sumatera Utara, Medan 20155,
Sumatera Utara, Indonesia
Keywords: Bioethanol, Bioethanol Gel, Carbopol, Saccharomyces Cerevisiae, Alternative Fuel.
Abstract: Bioethanol gel is an alternative fuel innovation with gel form, that ease packaging and distributing process.
Bioethanol gel is modified bioethanol with carbopol as the thickening agent. Based on the result of the study,
bioethanol gel as alternative fuel can be produced by hydrolyzing sugar cane waste and fermented with
Saccharomyces cerevisiae for 4 days, producing bioethanol gel with 95% grade. Carbopol as thickening agent
was added to bioethanol from sugar cane waste, producing bioethanol gel. Best result obtained with the
variation of carbopol 1,8 g and NaOH 1 mL resulted to flowing gel. Characteristics of bioethanol gel are: flare
time 237 seconds, residue 0,03 g, calorific value 33.064,25 kJ/kg, and 5 g of bioethanol.
1 INTRODUCTION
Indonesia is a country with high energy
consumption in the world. Energy source that take
the first place in consumption rank is petroleum
which is an non-renewable energy source. One of
the petroleum derivatives that are widely used in
small industries and households is kerosene
(paraffin).
At present government is trying to divert the use
of kerosene to other fuels, such as gas. But this
diversion has encountered many obstacles. For
example, the number of fire cases because leaked
gas from the tube. Therefore the conversion of
kerosene does not have to be to gas fuel but also to
another energy source, such as bioethanol which is
more environmentally friendly and does not
endanger the environment.
But bioethanol also has weakness in it's physical
properties. Bioethanol is volatile, have low surface
tension, and low flash points. Causing bioethanol in
liquid form can be dangerous (Robinson, 2006). For
this, we need to modify the form of bioethanol.
Bioethanol gel is a potential innovation for further
development. The gel form ease packaging and
distribution process. In production of bioethanol gel,
thickener is needed in the form of powder such as
calcium acetate, or other thickener such as xanthan
gum, carbopol and various cellulose derivative
materials (Tambunan, 2008).
Bioethanol gel has several advantages. They are
easy to handle, packed, and stored for it does not
easily spill and flow. Some advantages over other
fuels where during combustion are not smoky, do
not cause soot, and do not produce harmful gases.
Bioehtanol gel is non-carcinogenic and non-
corrosive (Merdjan and Matione, 2003). Bioethanol
gel innovation offers its own advantages over liquid
forms of biethanol both in terms of economy and
security. Based on research conducted by Hanun
(2018), states that bioethanol gel is more economical
than paraffin.
Bioethanol gel provides a solution to the safety
of the application of household energy use because it
does not easily spill and evaporate (Lloyd and
Visagie, 2007). Because bioethanol gel has more
advantages compared to liquid bioethanol, the
researchers hope to use bagasse as a source of
carbohydrates that can be fermented into bioethanol
and the addition of carbopol as gelling agent in the
production.
2 MATERIALS AND METHODS
2.1 Materials
The materials used in this study include: bagasse,
3.5% HNO
3
, NaNO
2
, 2% NaOH, 2% Na
2
SO
3
,
aquadest, 1.75% Na-Hypochlorite, 17.5% NaOH,
124
Az-zahra, W., Harahap, N., Putra, S. and Sinaga, M.
Production of Bioethanol Gel from Sugar Cane Waste with Carbopol as Alternative Fuel.
DOI: 10.5220/0008857501240129
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 124-129
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

iodine solution, HCl 30%, 10% NaOH, benedict
solution, MgSO
4
.7H
2
O, KH
2
PO
4
, (NH
4
)
2
SO
4
, bread
yeast, carbopol, NaOH 1N, soil oil, spritus and
gasoline.
2.2 Procedure
2.2.1 Bioethanol Making
Cellulose Isolation.
75 g of bagasse was putted into a beaker glass. Then
1000 mL of 3.5% HNO
3
and 10 mg NaNO
2
were
added. The mixture is heated by thermostat for 2
hours at 80°C. Then filtered and the residue was
washed with distilled water to pH = 7. To the
residue, 375 mL of 2% NaOH and 375 mL of 2%
Na
2
SO
3
was added. The mixture was heated by
thermostat for 1 hour at 50°C. Then it's filtered and
washed with distilled water to pH = 7. The residue
was added with 500 mL of Na-Hipochlorite 1.75%,
then heated using a thermostat for 30 minutes at
100°C which then was filtered and washed with
distilled water to pH = 7. 500 mL of NaOH 17.5%
was added and heated by thermostat for 30 minutes
at 80°C. Then filtered and washed the residue with
distilled water to pH = 7. To the residue was added
500 mL of 1.75% Na-Hipochlorite and heated for 5
minutes at 100°C. It was filtered and washed with
distilled water to pH = 7. After that the residue was
dried in the oven at 60°C then let it cooled down in
desiccator. Enough cellulose is putted onto a drip
plate and then dripped with iodine 0.1 solution
which will show positive cellulose test if there is no
color change and FTIR analysis is performed.
Cellulose Fermentation.
0.5 g of cellulose bagasse was putted into a 250 mL
erlenmeyer glass and 5 mL of distilled water was
added. 8 mL of 30% HCl was added to the mixture.
Erlenmeyer was covered with cotton and aluminum
foil before it was heated in thermostat at 80°C for 1
hour. The mixture was cooled to room temperature,
10% NaOH was added to get pH = 4–4.5, and then
filtered. 1 mL of filtrate was piped into a test tube
and 5 mL of Benedict's solution was added. It was
heated in a thermostat to form red brick deposits.
100 mL of glucose solution from hydrolysis of
bagasse was poured into a 250 mL Erlenmeyer glass.
0,1502 g of MgSO
4
.7H
2
O; 0,1306 g of KH
2
PO
4
; and
1,2021 g of (NH
4
)
2
SO
4
was added. The mixture was
sterilized using autoclave at 121°C for 1 hour and
then cooled. Bread yeast was added as much as 6
grams. Fermented for 2, 4, and 6 days. The
fermented product was then distilled at 78°C and
tested for ethanol using an alcohol hydrometer.
2.2.2 Making Bioethanol Gel
Thickener was added to bioethanol which has been
obtained from bagasse to form bioethanol gel. 100
mL of bioethanol was poured into a beaker glass and
stired with a speed of 1000 rpm, while 1.2 grams of
carbopol was added slowly. The glass beaker was
covered and the mixture was stirred continously for
45 minutes. Then 1 mL of 1 N NaOH is added to
form the bioethanol gel. The same experiment was
conducted with variation amount of carbopol (0.8 gr,
1 gr, 1.4 gr, 1.6 gr, and 1.8 gr) and 1 mL of NaOH.
2.3.3 Characterization
Stability and Flame Color Test for Bioethanol Gel.
5 grams of bioethanol gel was takken and putted into
a porcelain dish and then burned. The color and
flame of bioethanol gel combustion were observed
and recorded.
Ignition Time Test.
1 and 5 grams of bioethanol gel was putted into a
porcelain dish. Stopwatch was turned on when the
attempt to burn bioethanol gel was performed and
turned off when the flame appear.
Burned Bioetanol Gel Weight Test.
5 grams of bioethanol gel was putted into a porcelain
dish and then burned until the gel cannot burn again
(remaining ash and other solids). The residue then
weighed, where the weight of the bioethanol gel that
burns is the different between initial weight and final
weight.
Heat Test.
Testing of heat value refers to Robinson (2006),
carried out to determine the level of heat produced
by each sample of bioethanol gel in units of calories
(cal). The first step to measure the heating value
was, bioethanol gel was burned in the C200
Kalorimeter Bomb where the combustion product is
then cooled again to reach room temperature. The
energy used to cool combustion products is
equivalent to the energy available in fuel.
Movable Heat Test.
The transferred heat test or water boiling test was
done to determine the effectiveness of the fuel in
reference to Robinson (2006). Movable heat can be
measured by inserting 100 mL of water into a beaker
Production of Bioethanol Gel from Sugar Cane Waste with Carbopol as Alternative Fuel
125
glass and the initial temperature was measured. 15 g
of bioethanol gel was putted into a porcelain then
burned to heat 100 ml of water in a beaker glass.
After boiling water, the heat transferred is calculated
by calculating how much gel bioethanol is used for
this process.
3 RESULTS AND DISCUSSIONS
3.1 Bioethanol Making
3.1.1 Cellulose Isolation
Isolation of cellulose bagasse through several stages.
Starting from the delignification stage with addition
of HNO
3
and NaNO
2
, at this point impurities from
bagasse are removed and cellulose was produced.
Then proceed with pulping with NaOH and Na
2
SO
3
with 1: 2 ratio, this process is delignification
(removal of lignin) and will produce yellowish-
white cellulose. For the removal of dyes on
cellulose, bleaching with NaOCl is carried out.
Hypochlorite ions which are strong oxidants will
break ether bonds in lignin structure, consequently
the color of cellulose pulp becomes white. To
produce pure α-cellulose, 17.5% NaOH was added
to dissolve β-cellulose and produce α-yellowish
white cellulose. In other words, bleaching is needed
to produce white α-cellulose.
The result of cellulose isolation from bagasse is
white pulp, which 75g of bagasse is produced 10g α-
cellulose. The cellulose obtained was tested
qualitatively with iodine solution and showed
positive results with no color change.
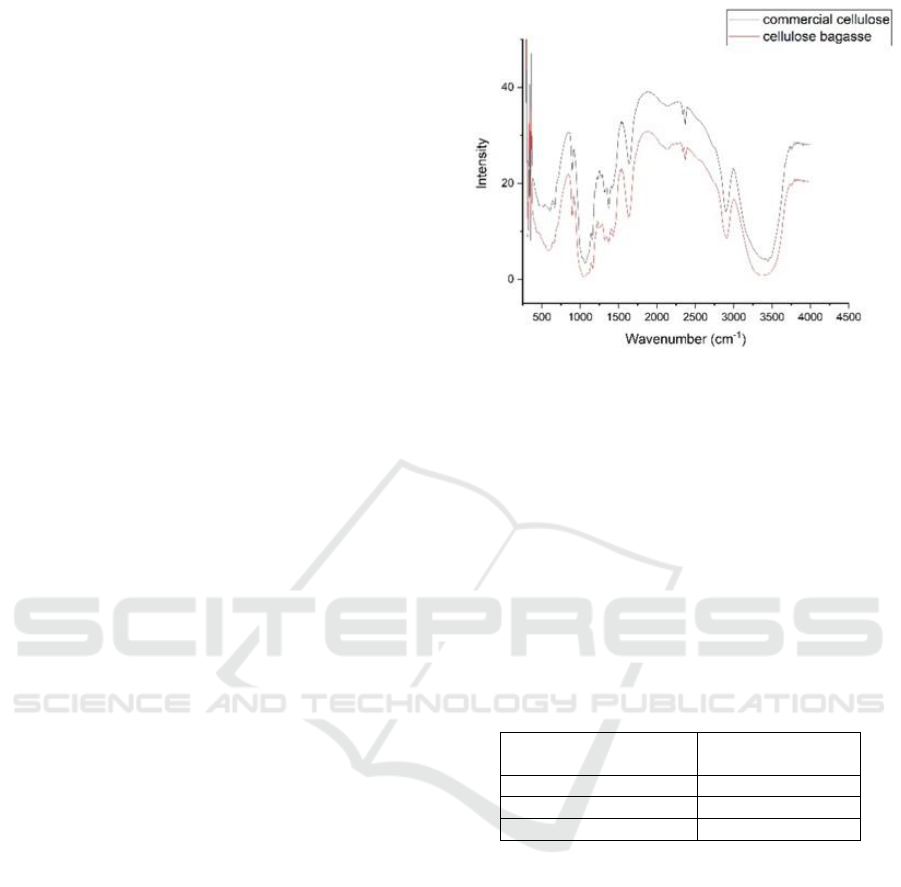
The FT-IR spectrophotometric test results also
showed positive results by comparing wave number
of bagasse cellulose and commercial cellulose.
Figure 1 is the result of FT-IR spectroscopic test
which shown spectrum with vibration peak in area
of 3448.72 cm
-1
for –OH group, supported by
emergence of vibrational peaks at wave number
2900.94 cm
-1
which shows C–H stretching groups,
1064, 71 cm
-1
which shows the ether group, and the
glycoside bond in α-cellulose structure is found at
wave number 1635.64 cm
-1
(Epriadi, 2017). Figure
1. showed that the results of FT-IR cellulose bagasse
had similar wave numbers with commercial
cellulose. Research on cellulose isolation has been
carried out by several researchers including: coconut
palm petiole (Xu et al., 2015), groundnut shells
(Bano and Negi, 2017), and corncob (Gea, 2019).
Figure 1: The spectrum of sugarcane pulp and commercial
FT-IR cellulose.
3.1.2 Cellulose Fermentation
Obtained cellulose bagasse pulp is hydrolyzed with
strong acid (HCl) for breaks the polymerization
chain of cellulose into a monomeric unit glucose.
The hydrolyzed cellulose is then neutralized by
adding NaOH to pH 4–4.5. Neutralization was done
to eliminate high residual acid from hydrolysis
process so that a standard product is obtained at pH
= 4–4.5. It is the optimum pH of Saccharomyces
cerevisiae growth (Oktavia, 2013).
Table 1: Cellulose fermented bagasse.
Fermentation Time
(Day)
Bioethanol Level
(%)
2
9,6
4
18,2
6
10,4
The data above shown optimum time to produce
bioethanol was until the 4th day. It's also shown in
Irvan (2015)'s study, that the optimum day to
produce bioethanol from bagasse was day 4 with 8
gram yeast producing 22.63% of ethanol.
The longer fermentation time, the more
Saccharomyces cerevisiae cells multiply and more
ethanol content is produced. This was a result of
longer time make more number of active bacteria,
multiply the ability to break substrate (Oktavia,
2013). However, on the 6th day Saccharomyces
cerevisiae has died and the substrate to be consumed
has been reduced.
Distillation in this study was carried out three
times. By using simple distillation, the distillation
will produce bioethanol for the first time with levels
of 10–20% and 50–70% at second attempt.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
126

Therefore, to get 90–95.5% levels, three repetitions
of distillation are performed. After being distilled,
the sample that was originally white will change
color to clear and the aroma of alcohol is smelled.
3.2 Making Bioethanol Gel
Bioethanol gel is produced by mixing with carbopol
and NaOH with slow stirring. The gel produced is
clear, in this case carbopol only acts as a gelling
agent without giving a color change to biethanol
(Wibowo, 2010). Addition of NaOH functions is to
neutralize acidic carbopol (Yogesthinaga, 2016).
The best bioethanol formulation is by adding 1.8
g of carbopol and 1 mL of NaOH. The form is
thicker than other variations. The bioethanol
produced can be seen in Figure 2.
Figure 2: Bioethanol gel.
3.3 Characterization
3.3.1 Stability and Flash Color Test for
Bioethanol Gel
According to Turns (2000), fire is a continuous heat
spread which is carried out by itself in a combustion
zone that is localized at very high speeds. One
characteristic of hydrocarbons combustion is
appearance of blue flashes in the zone of rapid
combustion in excess air conditions.
In the burning process, a good fire gives blue
color. Red color is produced due to incomplete
combustion process (Dewi, 2018). The flame of
bioethanol gel (Figure 3) is blue with an unstable
yellow to red tinge, and long-flaring flame. The red
tinge increases with addition of carbopol and NaOH
concentrations. According to Nugroho (2016), this
caused by combustion without intermediaries so the
fire seemed unstable. Turns (2000) also explained, a
long and flaming fire caused by combustion
conditions that are rich in fuel or the availability of
oxygen needed is not appropriate.
Figure 3: Bioethanol gel flame color.
3.3.2 Ignition Time Test
The combustion time test aims to see the ability of
bioethanol gel to burn until only part that cannot be
burned again remains and is calculated for a long
time until the fire is completely extinguished. The
results of measurement of combustion length of
bioethanol gel are presented in Table 2 and 3.
Table 2: Length of ignition test of 1 gram bioethanol gel.
Variation of
NaOH (mL)
Variation of
Carbopol (g)
Length of
Ignition (seconds)
1
0,8
88
1,0
95
1,2
101
1,4
109
1,6
120
1,8
180
Table 3: Length of ignition test of 1 gram bioethanol gel.
Variation of
NaOH (mL)
Variation of
Carbopol (g)
Length of
Ignition (seconds)
1
0,8
180
1,0
198
1,2
215
1,4
224
1,6
233
1,8
237
Data shown the best bioethanol gel burning time
is 239 seconds (3 minutes 59 seconds) on the
addition of 1.8 gram carbopol and 1 mL NaOH.
From data, it can be concluded that more carbopol
and NaOH additions able to withstand the burning
rate of bioethanol gel.
Presence of carbopol and NaOH is a retaining
factor so that the combustion becomes longer.
Increased concentration of carbopol extend the
flame because the vapor of bioethanol is trapped in
carbopol and released slowly, makes it run out
longer. When compared with liquid bioethanol, it
can be seen that bioethanol gel has increased the
Production of Bioethanol Gel from Sugar Cane Waste with Carbopol as Alternative Fuel
127
burning rate for a few seconds so it can be said that
the evaporation of bioethanol is inhibited by the
carbopol. Dewi (2018), has examined bioethanol gel
and get the same results; ignition period is increased
by addition of carbopol (1.5–2) grams.
3.3.3 Burned Bioetanol Gel Weight Test
This test is done to find out how much residue is
produced. Residue is a part of fuel that not
completely burn and left behind after the
combustion, changes, or reactions are complete. The
residual test results are presented in Table 4.
Table 4: Test for residual results.
Variation of
NaOH (mL)
Variation of
Carbopol (g)
Residue (g)
1
0,8
0,01
1,0
0,01
1,2
0,02
1,4
0,02
1,6
0,02
1,8
0,03
The remaining residue is the amount of
carbopol contained in the bioethanol gel which is
ensnared together with in the form of a gel, the dried
carbopol crust is brownish yellow. The data above
shown varies of residue produced in burning
process.
Wibowo (2010), stated that the more carbopol
added to 70% ethanol caused the residue to increase,
but this result was different in the bioethanol
treatment with 95% concentration. Based on the
results of the study, the increasing concentration of
ethanol produces fewer residues.
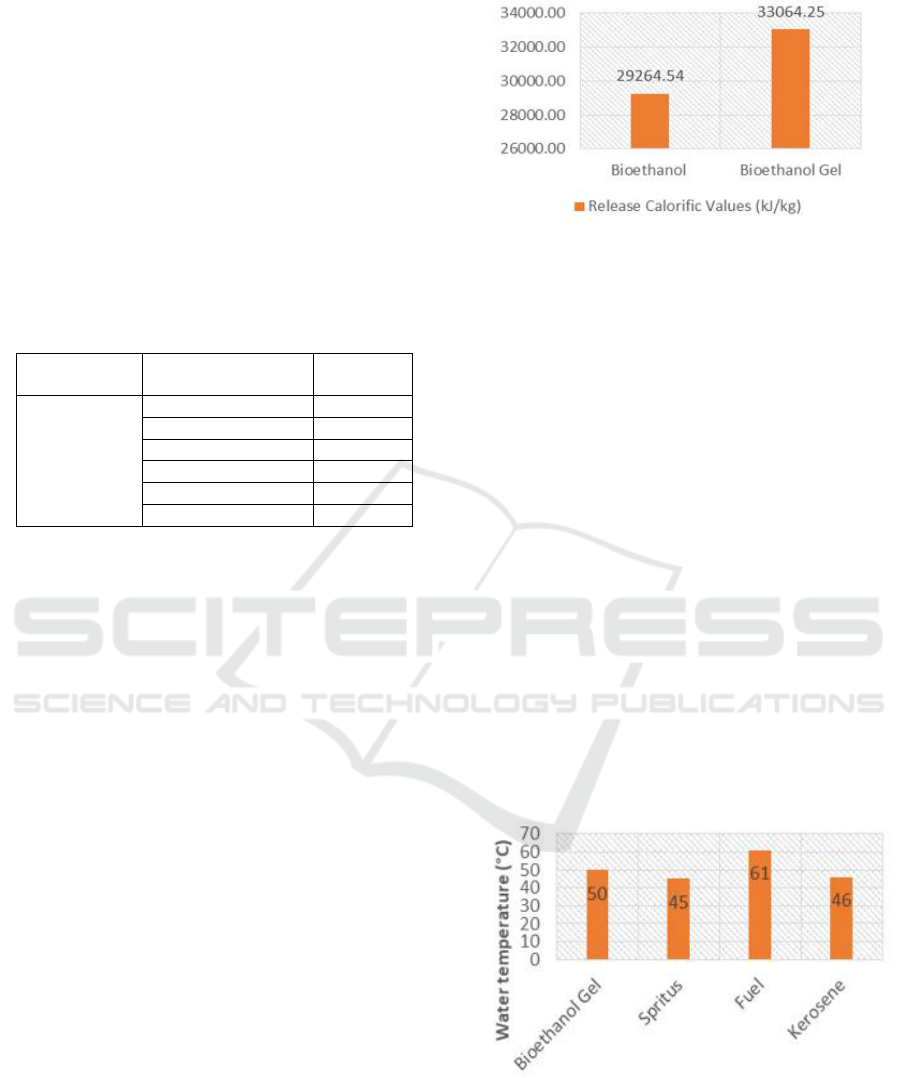
3.3.4 Heat Test
Calorific value is the most important quality
parameter for bioethanol gel as fuel. Calorific value
is the amount of heat energy stored in fuel produced
through combustion reactions. In fuel, the higher
calorie value possessed, the better quality of fuel and
higher combustion efficiency.
In this test, the best formulations were measured
from length of ignition. The calorific value obtained
from the measurement of the bioethanol gel calories
with the Calorimeter Bomb. The data are presented
in Figure 3.
Figure 3: Heat Value Test.
Calorific value is closely related to the
composition of carbon bound to a fuel. The higher
carbon bound gives higher calorific value (Yulistina,
2001 in Oktavia, 2013).
From the results of measurements using
Calorimeter Bomb, bioethanol gel heating value was
higher than liquid bioethanol. This is due to addition
of carbopol as a thickener, where carbopol is a
thickener of Lubrizol production with a molecular
formula (C
3
H
4
O
2
)
n
and has an active group of
polyacrylate acid that increased the calorifc value
(Dewi, 2018).
3.3.5 Movable Heat Test
A water boiling test is a test that determines the
performance of bioethanol gel so it can be used as a
household fuel. In this water boiling test, not all
bioethanol gel formulas were used, but the best
samples were taken, namely the addition of carbopol
1.8 g which was then compared with other fuels
such as: spritus, gasoline and kerosene. Heat transfer
data can be seen in Figure 4.
Figure 4: Heat transfer test.
In this test we use the same weight of all
materials, then measuring the temperature increased
from the heating process with the initial temperature
of water 25°C. The results of testing heat transfer
can be concluded that bioethanol gel is able to
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
128

increase the temperature better than spritus and
kerosene but not compared to gasoline. In addition,
bioethanol has other advantages, which are odorless
and do not cause soot in process. The practical use
of bioethanol gel is directly burned, unlike other
fuels that use intermediaries such as axes (Nugroho,
2016).
4 CONCLUSIONS
Based on the results of the research it can be
concluded that bioethanol gel as an alternative fuel
can be produced from hydrolysis of bagasse pulp
and then fermented with Saccharomyces cerevisiae
for 4 days and adding carbopol as thickener. The
best results were obtained with variations of
carbopol 1.8 g and 1 mL NaOH with gel flowing
forms. The characteristics of the bioethanol gel
were: flame length 239 seconds (3 minutes 59
seconds), residue 0.03 g, heating value 33.064,25 kJ
/ kg, and 5 g bioethanol gel can raise the water
temperature to 50 °C.
ACKNOWLEDGEMENTS
The authors would like to send gratitude to Risekti
dikti for the financial support towards this research
in the PKM-PE Project 2019 and also for
Universitas Sumatera Utara which facilitated this
research.
REFERENCES
Bano, S., Negi, Y. S., 2017. Studies on cellulose
nanocrystals isolated from groundnut shells.
Carbohydr. Polym. 157, 1041–1049.
Dewi, R. K., Poespowati, T., Jimmy, 2018. Natrosol
sebagai Salah Satu Bahan Pengental (Thickener) pada
Produksi Bioetanol Gel dari Limbah Daun Tebu.
Indones. Chem. Appl. J. 2, 1–6.
Epriadi, R., 2017. Isolasi Nanoserat Selulosa dari Tandan
Kosong Sawit (Elais guinensis jack) dengan
menggunakan Tempo. Universitas Sumatera Utara.
Gea, S., 2019. The Preparation of All-Cellulose
Nanocomposite Film from Isolated Cellulose of
Corncobs as Food Packaging The Preparation of
Cellulose Nanocomposite Film from.
Hanun, V., Sutjahjo, D., 2018. Komparasi karakteristik
bioetanol gel dengan pengental karbopol dan
carboxylmethyl cellulase (CMC) sebagai bahan bakar
alternatif. Tek. Mesin.
Irvan, Prawati, P., Trisakti, B., 2015. Pembuatan Bioetanol
dari Tepung Ampas Tebu Melalui Proses Hidrolisis
Termal dan Fermentasi: Pengaruh pH, Jenis Ragi dan
Waktu Fermentasi. Tek. Kim. USU 4, 27–31.
Lloyd, P. J. D., Visagie, E. M., 2007. A comparison of gel
fuels with alternative cooking fuels 18, 26–31.
Merdjan, R., Matione, J., 2003. Fuel Gel [WWW
Document]. United State Patents Appl. Publ.
Nugroho, A., Restuhadi, F., Rossi, E., 2016. Pembuatan
Gel Etanol denganMenggunakan Bahan Pengental
Carboxymethycellulose (CMC). Jom Faperta 3.
Oktavia, T., Sumiyati, S., Sutrisno, E., 2013. Pemanfaatan
Limbah Cair Cucian Beras sebagai Bahan Baku
Pembuatan Bioetanol Padat Secara Fermentasi oleh
Saccharomyces cerevisiae. Universitas Diponegoro.
Robinson, J., 2006. Bioethanol as a household cooking
fuel: a mini pilot study of the superblu stove in peri-
urban Malawi. Loughborough University.
Tambunan, L., 2008. Bioetanol Anti Tumpah 39, 24–25.
Turns, S., 2000. An Introduction To Combustion :
Concept And Applications, 2nd ed. McGraw-Hill
Book Company Singapore, Singapore.
Wibowo, W. A., Mulyono, T. S., 2010. Pembuatan dan
Uji Pembakaran Ethanol Gel. Ekuilibrium 9, 67–71.
Xu, C., Zhu, S., Xing, C., Li, D., Zhu, N., 2015. Isolation
and Properties of Cellulose Nanofibrils from Coconut
Palm Petioles by Different Mechanical Process 1–11.
Yogesthinaga, Y., 2016. Optimasi Gelling Agent Carbopol
dan Humektan Propilen Glikol dalam Formulasi
Sediaan Gel Ekstrak Etanol Daun Binahong (Anredera
cordifolia (Ten.) Steenis). Universitas Sanata Dharma.
Production of Bioethanol Gel from Sugar Cane Waste with Carbopol as Alternative Fuel
129
