
Phase Formation of M-Type BaFe
12
O
19
/ZnO Magnetic Material
Syahrul Humaidi
1*
, Tua Raja Simbolon
1
, Diana A Barus
1
, Veryyon Hrp
1
, Perdamean S
2
, Chandra K
2
,
Eko Arif S
2
and Silviana S
3
1
Department of Physics, Universitas Sumatera Utara, Jln Bioteknologi 1 Padang Bulan, Medan 20155, Indonesia
2
Research Center for Physics, Indonesian Intitute of Sciences (LIPI), Puspiptek, Serpong, TangSel, Indonesia
3
Mechanical Engineering, Universitas Pamulang, Jl. Rajawali Blok G No.33 Tangerang Selatan, Banten 15417
simbolonsilvi@gmail.com, candra.fisika.lipi@gmail.com, eko.arif.setiadi@lipi.go.id
Keywords: BaFe
12
O
19
, HEM, magnet.
Abstract: High Energy Milling (HEM) followed by heat treatment was used to prepare M-type BaFe
12
O
19
/ZnO magnetic
material in toluene media. High purity of BaFe
12
O
19
and ZnO powder was milled for 12 h followed by drying
for 4 h before being calcined at a temperature of 900
o
C. The powder was then filtered by using T-200. An
appropriate amount of BaFe12O19 and ZnO were then mixed in wet-milling for 15 mins and dried at 200
o
C
for 1 h. The magnetic properties were analysed using VSM, whereas the phase formation was derived from
XRD-pattern (powder method) using X-ray CuKα 40kV-30mA with λ= 1.5418 Å. The phase occurrence was
determined using MATCH-software program. It was obtained that BaFe
12
O
19
lattice parameter: a = 5.8930
Å, c = 23.1940 Å. In order to get soft magnetic state, ZnO was added with a composition of: 0/100, 25/100,
50/100, 75/100, respectively. It was found that addition of 75% ZnO to BaFe
12
O
19
converted hard magnet to
soft magnet.
1 INTRODUCTION
It has been known that the magnetic material
development is very vast in practical technical use
and industry application. Magnetic materials are used
in electronics, sensors, biomaterial as well as
transportation. Since 1950s, there were intensive and
extensive researches have been done. This kind of
material became interesting in the hexagonal ferrites,
known as hexaferrites [Pullar, 2012], which is
increasing exponentially until nowadays.
Barium hexaferrite (BaFe
12
O
19
) is a permanent
magnet based on ferrite (Ahmed et al, 2013; Yu,
2013). BaFe
12
O
19
is a type of M-ferrite hexagonal
(Ba-M) has some advantages compare to other
materials. The advantages of this material are: high
coersivity and high Curie temperature (An, 2014;
Burak, 2015), chemical stability, corrosion resistant,
high coersivity (Burak Kaynar, 2015). These good
characteristics make this M-type of magnet becomes
an interesting material to be developed.
Many methods of synthesis have been developed
over the laboratory and research centre over the world
to obtain a low production cost of powder particles of
barium hexaferrite. The scientists have been
developing many methods like powder metallurgy
method and chemical routes such as sol-gel method
as well as co-precipitation method (Setiadi et al,
2015). Setiadi (2018) also reported the application of
the powder magnetic material as Pb ion adsorbent.
Syahrul Humaidi (2015) has reported the role of Cu
2+
in BaFe
12-x
Cu
x
O
19
preparation.
Sintering process is widely used to modify the
characteristics of the magnet. It is common that some
additives may be used to enhance the characteristic of
the magnet such as silica (SiO
2
), Al
2
O
3
, Na
2
O, Fe
2
O
3
.
Not only dampen the grain growth, but also those
additives have the ability to serve lower the sintering
temperatures (Li et al., 2012). Supradedi et al (2017)
have investigated the addition of Na
2
O to BaFe
12
O
19
.
All of them reported that the composition of additive
Na
2
O at the sintering temperature 1200
o
C did not
influence the crystal structure. In this work,
BaFe
12
O
19
magnet was prepared by High Energy
Milling (HEM) method and ZnO was used as additive
to the phase formation of M-type BaFe
12
O
19
. The
main goal of the work is a change from hard magnetic
to soft magnetic.
Humaidi, S., Simbolon, T., Barus, D., Hrp, V., S, P., K, C., S, E. and S, S.
Phase Formation of M-Type BaFe12O19/ZnO Magnetic Material.
DOI: 10.5220/0008856701130115
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 113-115
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reser ved
113
2 MATERIALS AND METHODS
The preparation of the magnetic material using solid
state reaction method was started from preparation of
precursor: BaFe
12
O
19
powder and ZnO powder. At
first, barium ferrit as a matrix was mixed together
with ZnO powder in wet medium (toluene) and
experienced High Energy Milling (HEM) for 12 h.
The powder was then dried at 200
o
C for 4 h in oven
before sintering at 900
o
C for 3 h. Rigaku Smartlab
X-Ray Diffraction (XRD) Cu Kα (30kV, 40mA, λ =
1.5406 Å) was used to collect the maximum peaks
XRD-pattern. Analyse of the pattern was used by
MATCH program. Permagraph was used at room
temperature and normal atmosphere to get magnetic
properties in hysteresis curve form.
3 RESULTS AND DISCUSSION
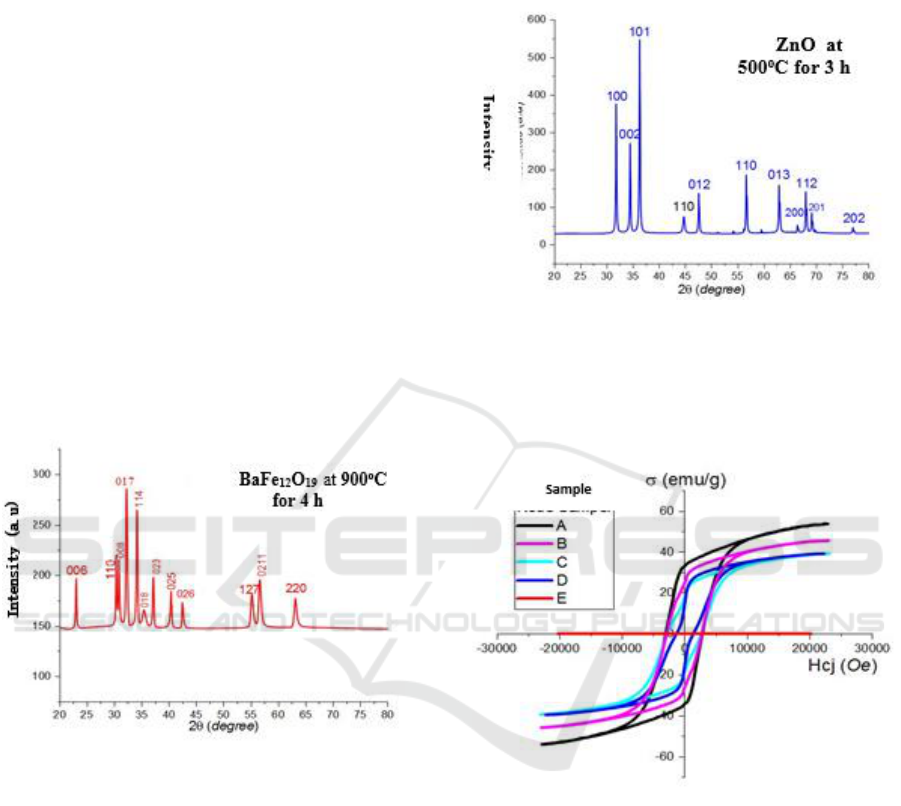
XRD-pattern of Ba-hexaferrit is shown in Figure 1,
whereas the ZnO phase is shown in Figure 2.
Figure 1: XRD -pattern of BaFe
12
O
19
at 900
o
C/4 h.
It can be seen clearly that four maximum peaks:
(110), (008), (017), and (114) occur in 30
o
< 2θ < 40
o
.
The occurrence of these peaks indicate that the phase
occurrence corresponds to BaFe
12
O
19
structure as a
major phase. This results are in a good agreement
with the previous finding (Syahrul Humaidi, 2015).
This sample has a hexagonal crystalline structure
whose space group (P63/mmc) with lattice parameter:
a = 5.8930Å and c=23.1940 Å. Preparation of ZnO
precursor with sintering temperature at 500
o
C as
shown in Figure 2 also a confirmation of ZnO as a
major phase. It can be seen that three maximum
peaks: (100) related to 2θ= 31.80
o
; (002) at 2θ= 31.8
o
and (101) at 2θ= 34.42
o
. According to the results of
MATCH program, the crystalline structure of ZnO is
hexagonal with the value of a parameter = 3.22Å and
b= 5.22Å space group of P63mc (wurtzite). As such,
ZnO can be used as a filler to barium-hexaferrit
magnet.
Figure 2: XRD -pattern of ZnO at 500
o
C/3 h.
Figure 3 tells magnetic characteristics of produced
composite magnet. Based on this graph, the value of
magnetic remanence (Mr) as well as coersivity (Hcj)
can be gained as tabulated in Table 1.
The curve style as presented in Figure 3 starts to
deviate in the composition of (50/50) % weight of the
precursor. A narrower curve can be observed in the
composition of (25/75) % weight of ZnO. Based on
the graphs, it can be concluded that the addition of
75%-weight of ZnO has turned the hard magnetic into
soft magnetic. The value of soft magnetic properties
can be referred on Table 1. As it can be seen, the
smallest value of Mr as well as the Hcj have been
noted in the sample C. However, further investigation
is still needed to confirm this phenomena. Based on
this result, this composition (25% BaFe
12
O
19
+ 75%
ZnO) is reasonable to be developed as a starting
material composition especially in the application of
microwave absorber.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
114
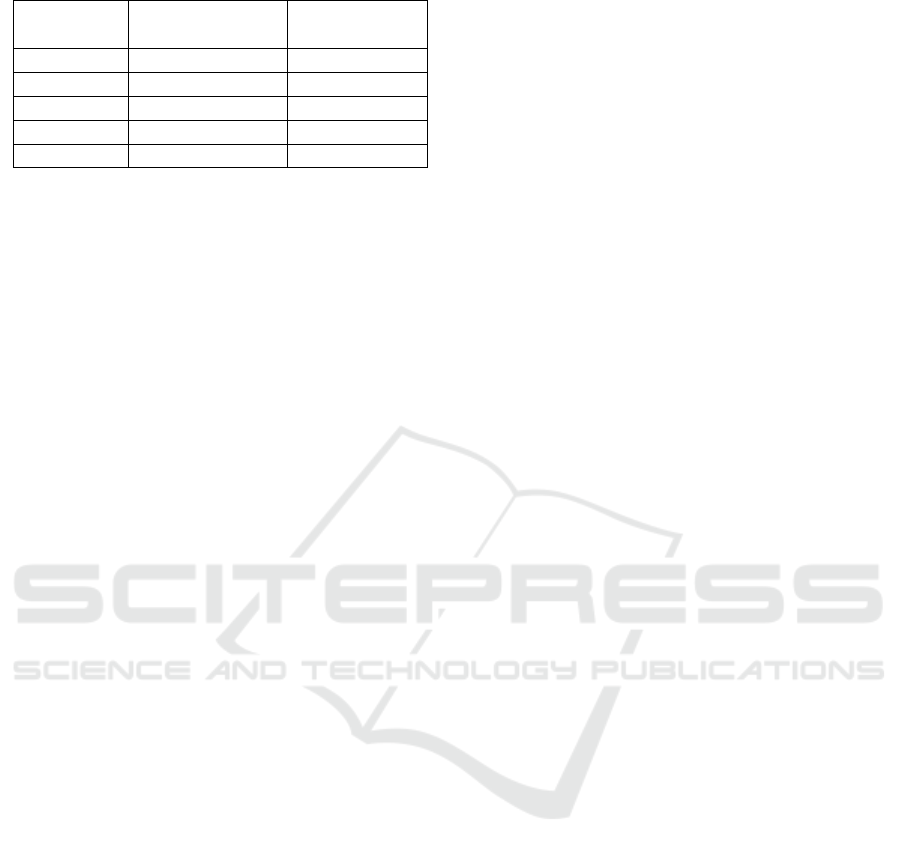
Table 1: Magnetic properties of composite magnet
Sample
code
Mr
(emu/g)
Hcj
(Oe)
A (100/0)
33.06
2943
B (75/25)
24.60
2821
C (50/50)
17.20
2728
D (25/75)
1.27
152.4
E (0/100)
14.69
1365
4 CONCLUSIONS
We have successful prepare a composite magnetic
material BaFe
12
O
19
/ZnO via solid state reaction. The
composition of 25/75 can be referred as a starting
composition in the next soft magnetic investigation.
ACKNOWLEDGEMENTS
We would like to thanks members of Physics
Research Centre (P2F) Serpong for sample
preparation and characterizations.
REFERENCES
Pullar, R., C., 2012, Hexagonal Ferrites: A review of the
Synthesis, Proerties, and Applications of Hexaferrite
Ceramics, Progress in Materials Science, vol 57, Issue
7, pp 1191-1334
An, G. H., Hwang, T. Y., Kim, J., Kim, J. B., Kang, N.,
Kim, S., Choi, Y. M., Choa, Y. H., 2014, Barium
hexaferrite nano particles with high magnetic properties
salt-assisted ultrasonic spray pyrolysis. Journal of
Alloys and Compounds 583 145-150.
Setiadi E. A., Yunus M, Nababan N , Simbolon S ,
Kurniawan C , Humaidi S, Sebayang P and Ginting M,
2018, The effect of temperature on synthesis of
MgFe2O4 based on natural iron sand by co-
precipitation method as adsorbent Pb ion, Journal of
Physics: Conf. Series 985 012046
Burak Kaynar, M., Şadan, Özcan S. Ismat Shah, 2015,
Synthesis and magnetic properties of nanocrystalline
BaFe12O19, Ceramics International, Vol.41, Issue 9,
Part A, Pages 11257-11263
Ahmed, M., A., Helmy, N., El-Dek, S. I., 2013, Innovative
Methodology for the Synthesis of Ba-M hexaferrite
BaFe12O19 nanoparticles Materials Research Bulletin
48, 3394-3398
Yu, H., F., 2013, BaFe12O19 powder with high
magnetization prepared by acetone-aided
coprecipitation. Journal of Magnetism and Magnetic
Materials 341, 79-85
Syahrul Humaidi, Ratna AS, Tua Raja S, Sri Dermayu S,
Perdamean S, 2015, Magnetic Properties of Cu2+
Substituted BaFe12-xCuxO19, Indonesian Journal of
Applied Physics vol.5 no.1 pp 71-78
Supradedi, P., Sardjono, Muljadi, N., Rusnaeni and
Humaidi, S., 2017, Effect of additive Na2O on sintering
temperature, crystal structure and magnetic properties
of BaFe12O19 magnet, Journal of Physics: Conf. Ser.
817 012056
Phase Formation of M-Type BaFe12O19/ZnO Magnetic Material
115
