
Phosphate Adsorption using KOH Activated Coal Bottom Ash
Fatimah Agussalim, Alfikri Ramadhan, Budi Pratama Tarigan
Chemical Engineering Department, Engineering Faculty, Universitas Sumatera Utara, Medan, Indonesia, 20155
Keywords: Adsorption, Adsorption capacity, Bottom ash, Freundlich isotherm model.
Abstract: Research on phosphate adsorption using activated KOH coal bottom ash has been carried out. This study aims
to assess the utilization of bottom ash as an adsorbent to adsorb phosphate ions from water bodies. Research
starts from the activation process for 5 hours with 3 M NaOH solution. Then the pre-activated and post-
activated bottom ash are characterized using Scanning Electron Microscopy-Energy Dispersive X-ray
Spectroscopy (SEM-EDS). Based on the results of the characterization with SEM-EDS, the surface of pre-
activated bottom ash surfaces seems smooth because they are still covered by impurity metals such as Fe, Ti
and Mg. After activation, bottom ash surfaces become rough because impurities such as Fe, Ti, and Mg are
lost and thus the pores are opened. The bottom ash then was applied to absorb phosphate ions with variations
of particle size (50-70 mesh, 70-110 mesh, and 110-140 mesh) and dosage of adsorbent (1, 2, and 3 g) and
were contacted for 60 minutes to 1000 ml phosphate solute of concentration 10 mg/l. Remaining phosphate
ions concentration in solute after adsorption are analyzed using UV-vis spectrophotometer. Best adsorption
of phosphate ions with 74.8% efficiency was obtained at particle size of 110-140 mesh and dosage adsorbent
of 3 g. The highest adsorption capacity (7.02 mg / g) was obtained with dosage of 1 g adsorbent. Freundlich
and Langmuir's models were used to describe the phosphate ion adsorption by KOH activated bottom ash
isotherm. Based on the data obtained, the suitable model for this study is Freundlich model with a value of R
2
= 0.9721.
1 INTRODUCTION
Phosphate is a nutrient that is very important for life.
Phosphate is a key component added in making
fertilizers to produce food (Ashley et al, 2011;
Cordell et al, 2009). However, the presence of
phosphate with a range of more than 0.01-0.1 mg/L
in a body of water can trigger a eutrophication process
which will reduce the penetration of sunlight into the
body of water during the day so that photosynthesis
that will produce oxygen is also reduced (Kumar et
al, 2019).
The phenomenon of eutrophication can trigger
the growth of algae and microorganisms so that the
waters become green, turbid, odourless and reduce
oxygen levels (Ajmal et al, 2018). There are several
ways that have been successful in reducing phosphate
levels in the body of water, namely biological
treatment (Peng el al, 2016), chemical precipitation
(Van der Houwen et al, 2001), struvite formation
(Muhmood et al, 2018), membrane processing
(Peleka, 2006), and adsorption (Ghaneian et al, 2014;
Usman et al, 2018). The adsorption method has
several advantages compared to other methods, which
are simple, inexpensive, and high efficiency (Seftel et
al, 2018).
Bottom ash from burning coal PT. SOCIMAS was
used as an adsorbent in this study. Bottom ash has a
high particle size, surface area and porosity making it
a good choice for use as an adsorbent (Gorme et al,
2010). Bottom ash has a large Si and Al content that
makes bottom ash can be used as zeolite or adsorbent
(Bertolini et al, 2013). Some previous studies have
used bottom ash as an adsorbent and its absorption
efficiency is quite high (Gorme et al, 2010; Bertolini
et al, 2013; Gandhimathi et al, 2013; Mittal et al,
2013., Dincer et al, 2007; Saleh et al, 2012)
This study aims to examine the potential of
bottom ash which has been activated with KOH as an
adsorbent by looking at the effect of particle size and
dosage of adsorbent usage. The Langmuir and
Freundlich isotherm models were used to model the
isotherm data for their applicability.
100
Agussalim, F., Ramadhan, A. and Tarigan, B.
Phosphate Adsorption using KOH Activated Coal Bottom Ash.
DOI: 10.5220/0008855501000104
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 100-104
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2 RESEARCH METHOD
The activation of bottom ash using KOH solute has
been held with the following procedures: dissolve 30
gr of bottom ash in 250 ml KOH solute of 3 M, stir
with stirring rate of 200 rpm for 5 hours at
temperature of 85-95
o
C. The mixture then washed,
filtered, and dried in oven at temperature of 105
o
C.
The structure and composition of pre-activated and
activated bottom ash was analyzed using SEM-EDS.
A batch experiment was performed by taking a
specified amount of adsorbent and combining it with
1000 ml phosphate solution at concentration of 10
mg/l. After adsorption, the concentration of
phosphate was determined using a spectrophotometer
UV-Vis with SNI 06-6989.31-2005 method.
In order to investigate the effect of particle size on
phosphate adsorption, three different sizes of
adsorbent (between 50 and 70 mesh, between 70 and
110 mesh, and between 110 and 140 mesh) and
constant adsorbent dosage (1 g) was used. To observe
the effect of adsorbent dosage on phosphate removal
and adsorption capacity, three different adsorbent
dosage (1, 2, and 3 g) were used. In order to determine
the fitting isotherm model, the initial concentrations
of adsorbate was varied accordingly (2, 4, 6, 8, and
10 mg/l).
3 RESULTS AND DISCUSSION
3.1 Effect of Activation
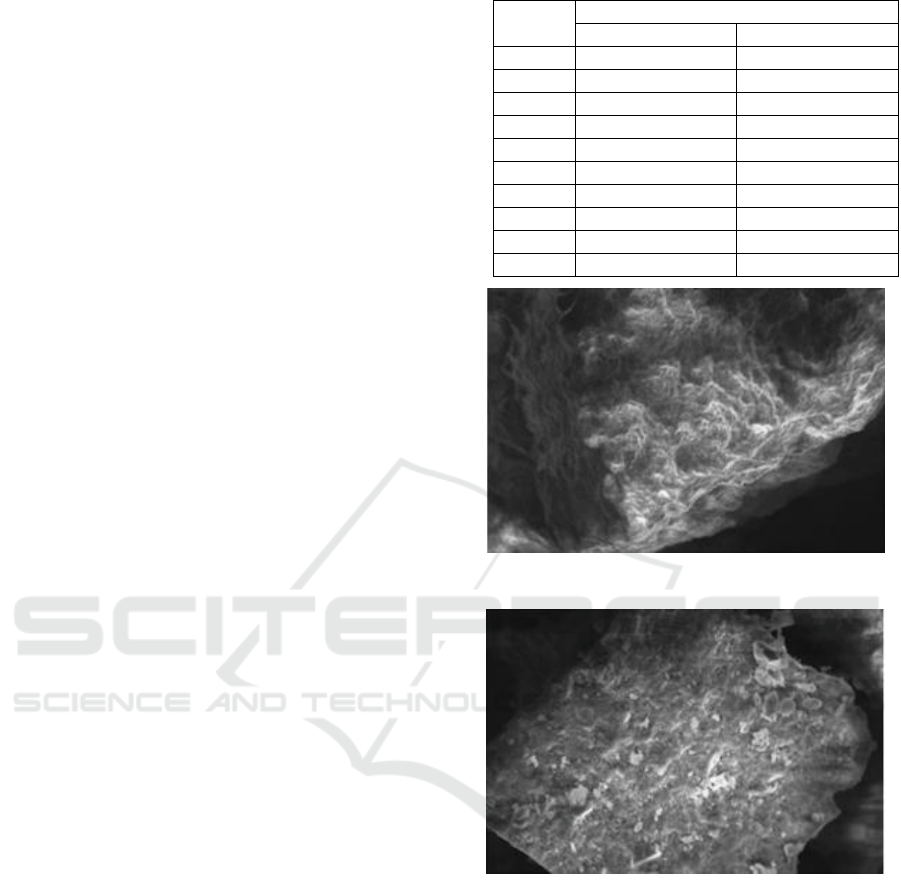
The effect of activation is shown in Fig. 1 and 2. As
shows in Fig. 1, bottom ash before activation doesn’t
have pores because covers by impurities and Fig. 2,
bottom ash after activation have more much pores
because bottom ash after activation have fewer
impurities than bottom ash before activation. This can
make bottom ash has bigger adsorption capacity
(Bronislaw, 2016).
Similar result was observed in a study of overview
on the effect of activation on the performance of
graphene based adsorbent by Jie, et al. (Ma, 2017).
According to cited authors, adsorbent with more
much pores increase the amount of adsorbate which
could adsorbed.
The activation process also change the chemical
composition of bottom ash. Activation process can
eliminate impurities in the bottom ash. In Table 1, it
can be seen elements such as Fe, Mg, and Ti are lost
after the activation process. The lost elements can
provide additional pores for adsorption process.
Table 1: Chemical composition of bottom ash before and
after activation
Element
Percentage
Before activation
After activation
O
47.67
48.79
Si
19.59
13.92
Al
8.58
12.69
Br
15.13
16.44
K
0.61
6.25
Ca
1.49
1.18
Na
2.07
0.73
Fe
3.08
-
Mg
1.08
-
Ti
0.70
-
Figure 1: SEM bottom ash result before activation
Figure 2: SEM bottom ash result after activation
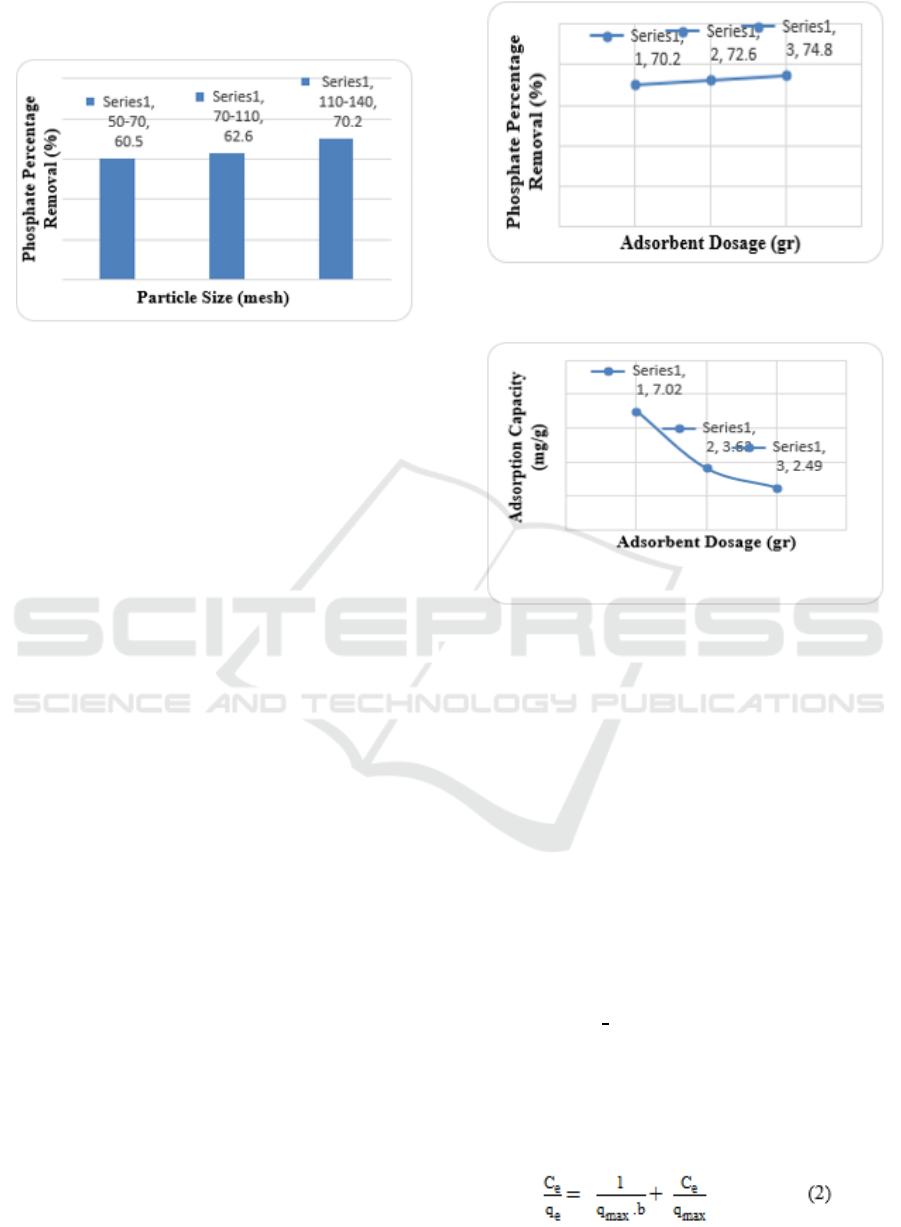
3.2 Effect of Particle Size
The effect of particle size is shown in Fig. 1. As
shown in Fig. 1, the phosphate removal percentage
increase as the particle size was decreased. The
highest phosphate percentage removal (70.2 %) was
achieved by the adsorbent of particle size between
110 and 140 mesh.
Similar result was observed in a study of overview
on the effect of particle size on the performace of
wood-based adsorbent by Ikenyiri and Ukpaka
(Ikenyiri, 2016). According to cited authors,
adsorbent with smaller particle size has higher surface
Phosphate Adsorption using KOH Activated Coal Bottom Ash
101
area, thus increase the amount of adsorbate which
could adsorbed.
Figure 3: Phosphate percentage removal at three different
particle size
3.3 Effect of Adsorbent Dosage
The effect of adsorbent dosage on phosphate
adsorption is shown in Fig. 2 and 3. It was observed
that phosphate percentage removal increase with an
increase of adsorbant dosage. In contrast, adsorption
capacity decrease with an increase of adsorbent
dosage. The highest adsorption capacity (7,02 mg/g)
was achieved with adsorbent dosage of 1 gr. The
similar trend was reported by Hasfalina, et al
(Hasfalina et al, 2012). Futher increment of adsorbent
dosage above 1 g/L resulted in a decline in adsorption
capacity.
Charles and Odoemelam had stated in their study
that an increase in adsorbent dosage will provide
greater availability of the exchangeable sites or
surface area (Charles et al, 2010). Hence, the
percentage of phosphate removal in this study
increases as the adsorbent dosage increases (Fig. 2).
However, the increase is not significant and higher
adsorbent dosage does not provide higher adsorption
capacity (Fig. 3). The trend where phosphate
adsorption capacity descreases as adsorbent dosage
increases in this study might be due to the adsorptive
capacity of bottom ash that was available was not
fully utilized at higher adsorbent dosage in
comparison to lower adsorbent dosage.
Figure 4: Phosphate percentage removal at different
adsorbent dosage
Figure 5: Phosphate adsorption capacity at different
adsorbent dosage
According to Waseem et al, high adsorbent dosage
does not always gives optimum result and higher
adsorption capacity compared to lower dosage
(Waseem et al, 2012). This is possibly because of at
higher adsorbent dosage, lumps was formed and thus
decrease overall surface area of adsorbent.
3.4 Adsorption Isotherm Model
The Freudlich and Langmuir adsorption models were
used to determine the fitting model for phosphate
adsorption using KOH activated bottom ash. The
Freudlich and Langmuir isotherm are given in Eq. 1
and 2, respectively (Ahmed et al, 2010).
log q
e
= log K
f
+
1
n
log C
e
(1)
Where qe is the amount of phosphate adsorbed per
mass adsorbent used (mg/g), Ce is equilibrium
concentrate of solution (mg/l), Kf is Freudlich
constants and 1/n is adsorption intensity.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
102

Where q
max
is adsorption capacity at monolayer, b
is Langmuir constants, qe is the amount of phosphate
adsorbed per mass adsorbent used (mg/g), and Ce is
equilibrium concentrate of solution (mg/l).
The experimental data was applied to the isotherm
models, and corresponding plots are given in Fig. 4
and 5.
Figure 6: Freudich plot for phosphate removal
Figure 7: Langmuir plot for phosphate removal
Based on Fig. 6 and 7, a summary table was made
and shown in Table 2.
Table 2: Freudlich and Langmuir Constants
Parameter
Freudlich
Langmuir
R
2
0.9512
0.8171
Q
max
-
9.149
K
2.671
0.662
n
1.194
-
In Table 2, the values of R2 were compared. The
R
2
value of Freudlich model (0.9512) is well fitted
compared to the R
2
value of Langmuir model
(0.8171). This is indicating that the mechanism of
phosphate adsorption using KOH activated bottom
ash obeyed the Freudlich isotherms. The good fit of
the Freudlich isotherm to an adsorption system
assumes tha there is a multilayer adsorption and
reversible adsorption which considers the interaction
between adsorbate molucules (Freundlich, 1906).
4 CONCLUSIONS
In general, it can be concluded that the parameters
such as particle size and adsorbent dosage did affect
the adsorption of phosphate removal using KOH
activated bottom ash. The highest percentage removal
value of 70.2 % was achieved by using adsorbent with
particle size between 110 and 140 mesh. Higher
adsorbent dosage gives a decrease in adsorption
capacity, with the highest value of adsorption
capacity was achieved by using adsorbent dosage of
1 gr. Equilibrium data by the Freudlich isotherms (R
2
= 0.9512) was found to be well fitted in describing
adsorption isotherm of KOH activated bottom ash for
phosphate removal.
ACKNOWLEDGEMENTS
The authors would like to express thanks for the
support of Chemical Engineering Department of
Universitas Sumatera Utara for providing research
facilities.
REFERENCES
Ahmed, Y.M., Abdullah Al-Mamun, Suleyman Aremu
Muyibi, Ma’an Fahmi R., Al-Khatib, Ahmed Tariq
Jameel, and Mohammed A., Al Saadi., 2010. Effect of
Adsorbate Initial Concentration on the Removal of Pb
from Aqueous Solutions by Carbon Nanofibers.
Nanoscience and Nanotechnology Research Group
(NANORG), Malaysia.
Ajmal, Z., Muhmood, A., Usman, M., Kizito, S., Lu, J.,
Dong, R., Wu, S., 2018. Phosphate Removal from
Aqueous Solution Using Iron Oxides: Adsorption,
Desorption and Regeneration Characteristics. Journal
of Colloid and Interface Science.
Ashley, K., Cordell, D., Mavinic, D., 2011, A Brief History
of Phosphorus: From The Philosopher's Stone to
Nutrient Recovery and Reuse. Chemosphere 84 737–
746.
Babayemi, Kamoru, A., Onukwuli, Dominic. O., 2017.
Equilibrium Studies and Optimization of Phosphate
Adsorption from Synthettic Effluent Using Acid
Modified Bio-Sorbent. American Journal of
Engineering and Applied Sciences 2017, 10 (4) :
980.991.
Bertolini, T.C.R., Izidoro, J.C., Magdalena, C.P., Fungaro,
D.A., 2013.. Adsorption of Crystal Violet Dye from
Phosphate Adsorption using KOH Activated Coal Bottom Ash
103

Aqueous Solution onto Zeolites from Coal Fly and
Bottom Ashes. Orbital: Electron. J. Chem. 5 (3):179-
191.
Bronislaw Buczek., 2016. Preparation of Active Carbon by
Additional Activation with Potassium Hydroxide and
Characterization of Their Properties. Advances in
Materials Science and Engineering, Volume 2016,
Article ID 5819208, 4 pages.
Charles, Osu., and Odoemelam, S.A., 2010. Study on
Adsorbent Dosage, Particle Sizes and Ph Constraints on
Biosorption of Pb (II) and Cd (II) Ions from Aqueous
Solution Using Modified and Unmodified Crasstrotrea
Gasar (Bivalve) Biomass. International Archive of
Applied Sciences and Technology, Vol. 1 (1) June
2010: 62-68 (2010).
Cordell, D., Drangert, J. O., White, S., 2009. The Story of
Phosphorus: Global Food Security and Food for
Thought. Global Environmental Change 19 (2009)
292–305.
Dincer, A.R., Gunes¸ Y., Karakaya, N., 2007. Coal-based
Bottom Ash (CBBA) Waste Material as Adsorbent for
Removal of Textile Dyestuffs from Aqueous Solution.
Journal of Hazardous Materials 141 529–535.
Freundlich, H. M. F., 1906. Uber die Adsorption in
Losungen. Zeithschrift fur Physikalische Chemie
(Leipzig). 57A, 385-470.
Gandhimathi, R., Ramesh, S.T., Sindhu, V., Nidheesh,
P.V., 2013.. Bottom ash Adsorption of Basic Dyes from
Their Binary Aqueous Solutions. Songklanakarin J. Sci.
Technol. 35 (3), 339-347.
Ghaneian, M.T., Ghanizadeh, G., Alizadeh, M.T.H.,
Ehrampoush, M.H., Said, F.M., 2014. Equilibrium and
Kinetics of Phosphorous Adsorption onto Bone
Charcoal from Aqueous Solution. Environ Technol. 35
882-890.
Gorme, J.B., Maniquiz, M.C., Kim, S.S., Son, Y.G., Kim,
Y.T., Kim, L.H., 2010. Characterization of Bottom Ash
as an Adsorbent of Lead from Aqueous Solutions.
Environ. Eng. Res.15(4) : 207-213.
Hasfalina, Man, C., Chin, W.H., Zadeh, M.R., Rashid, M.,
Yusof., M., 2012. Adsorption Potential of Unmodified
Rice Husk for Boron Removal. Bioresources 7 (3),
3810-3822.
Ikenyiri PN., Ukpaka CP., 2016. Overview on the Effect of
Particle Size on the Performance of Wood Based
Adsorbent. J Chem Eng Process Technol 7: 315. doi:
10.4172/2157-7048.1000315.
Kumar, P.S., Korving, L., Keesman, K.J., M.C.M. van
Loosdrecht, M.CM., 2019. Eff ect of Pore Size
Distribution and Particle Size of Porous Metal Oxides
on Phosphate Adsorption Capacity and Kinetics.
Chemical Engineering Journal 358 (2019) 160–169.
Ma J., Sun Y., Yu F., 2017. Efficient Removal of
Tetracycline with KOH-Activated Graphene from
Aqueous Solution. R. Soc. open sci. 4: 170731.
Mittal, J., Jhare, D., Vardhan, H., Mittal, A., 2013.
Desalination and Water Treatment : Utilization of
Bottom Ash as a Low-Cost Sorbent for the Removal
and Recovery of a Toxic Halogen Containing Dye
Eosin Yellow. Desalination and Water Treatment, DOI:
10.1080/19443994.2013.803265.
Muhmood, A., Wu, S., Lu, J., Ajmal, Z., Luo, H., Dong,
R., 2018. Nutrient Recovery from Anaerobically
Digested Chicken Slurry via Struvite: Performance
Optimization and Interactions with Heavy Metals and
Pathogens. Sci. Total Environ. 635 1-9.
Onar, A. Nur, Balkava, N., Akyuz. T., 1996. Phosphate
Removal by Adsorption. Enviromental Technology,
Vol. 17 pp 207-2013, Publications Division Selper Ltd.
Peleka, E.N., Mavros, P.P., Zamboulis, D., Matis, K.A.,
2006. Removal of Phosphates from Water by a Hybrid
Flotation–Membrane Filtration Cell. Desalination 198
198-207.
Peng, Y.Z., Wang, X.L., Li, B.K., 2006. Anoxic Biological
Phosphorus Uptake and the Effect of Excessive
Aeration On Biological Phosphorus Removal In The
A(2)O Process. Desalination 189(1-3) 155-164.
Saleh, S.M., Maarof, H.I., Rahim, S.N.S.A., Nasuha, N.,
2012.. Adsorption Congo Red into Bottom Ash. Journal
Of Applied Sciences 12 (11): 1181-1185.
Seftel, E.M., Ciocarlan, R.G., Michielsen, Meynen, V.,
Mullens, S., Cool, P., 2018. Insights into Phosphate
Adsorption Behavior on Structurally Modified ZnAl
Layered Double Hydroxides. Applied Clay Science 165
234–246.
Siwek, Hanna, Artur Bartkowiak, and Malgorzata
Wlodarczyk., 2019. Adsorption of Phosphates from
Aqueous Solutions on Alginate/Goethite Hydrogel
Composite. Water 2019, 11, 633;
doi:10.3390/w11040633.
Usman, M., Byrne, J.M., Chaudhary, A., Orsetti, S., Hanna,
K., Ruby, C., Kappler, A., Haderlein, S.B., 2018.
Magnetite and Green Rust: Synthesis, Properties, and
Environmental Applications of Mixed-Valent Iron
Minerals. Chem. Rev. 118(7) 3251-3304.
Van der Houwen, J., Valsami-Jones, E., 2001. The
Application of Calcium Phosphate Precipitation
Chemistry to Phosphorus Recovery: The Influence of
Organic Ligands Environ. Technol. 22 1325-1335.
Waseem, S., Din, M.I., Naseer, S.,. Rasool, A., 2012.
Evaluation of Acacia Nilotica as a Non Conventional
Low Cost Biosorbent for the Elimination of Pb (II) and
Cd (II) Ions from Aqueous Solutions. Arabian Journal
of Chemistry, VII.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
104
