
Pillarization of Bentonite using Fe
3+
/Ti
4+
and Its Application for
Congo Red and Direct Violet Removal
Muhammad Said
*
, Riza Antini, Tarmizi Taher, Aldes Lesbani
Department of Chemistry, Sriwijaya University, Jalan Palembang-Prabumuih Km.32, Indralaya, Indonesia
Keywords: Bentonite, Pillarization, Fe
3+
/Ti
4+
, Congo red, Direct Violet, Adsorption.
Abstract: Bentonite was pillarized by using Fe
3+
/Ti
4+
metal oxide ratio 2:1 and 1:2. The pillarization product was
characterized by using XRD analysis and FT-IR spectrophotometer. XRD diffractogram showed that the
optimal product was obtained at Fe
3+
/Ti
4+
ratio 2:1. At this ratio, basal spacing of bentonite layer is 15 Å
while bentonite with ratio 1:2 has basal spacing 12.86 Å. Bentonite 2:1 ratio was applied on Congo red
adsorption. The isotherm adsorption was calculated and followed Freundlich model having adsorption rate
(k) 0.0008 (min
-1
) for Congo red and 0.00045 (min
-1
) for direct violet. The adsorption capacity (qe) of
Congo red at 70 °C is 68 mol/g that larger than 51 mol/g for direct violet. The adsorption energy (E) of
Congo red at 70°C is 4.90 kJ/mol also higher then direct violet with 1.01 kJ/mol. The enthalpy (ΔH) and
entropy (ΔS) is decreased with increase of Congo red and direct violet concentration. The optimum pH was
obtained at 3 whereas the maximum amount of Congo red adsorbed is 90.60 mg/L. Direct violet shows
maximum amount of adsorption at pH 4 with 79.61 mg/L.
1 INTRODUCTION
Bentonite is a naturally occurring material with
layered structure contains inorganic exchangeable
cations (Kaufhold and Dohrmann, 2008). It is also
known as clay mineral composed predominantly by
montmorillonite ~85% (Martin et al., 2019) with
general formula (OH)
4
Si
8
Al
4
O
20
.nH
2
O (Hao et al.,
2014). The exchangeable cations contain within
layer structure of bentonite in addition to swelling
ability made this material suitable for adsorbent and
catalyst application (Sahara, 2010).
Natural bentonite has been used in direct
application but shows low effectively due to small
inter-layer distance and unreliable porosity.
(Goodarzi et al., 2016). Efforts must be introduced
to enhance natural bentonite quality through various
methods such as pillarization. Pillarization involves
ions, molecules or compounds insertion in the
interlayer of bentonite (Okoye and Obi, 2011). In
this research, We used macro-anion of Fe
3+
/Ti
4+
.
Pillarization result has the advantage of larger
distance of inter-layer with a steady or permanent
porosity (Cool and Vansant, 1998).
Fe
3+
/Ti
4+
Pillared bentonite can be applied as
adsorbent for removal of Congo red and direct violet
dyes. Both dyes has azo group (R-N=N-R) and as
typical of synthetic dye, it is toxic and hard to
degrade due to its complex chemical structure which
contains aromatic rings (Unuabonah et al., 2008).
The adsorption process was conducted by using
Fe
3+
/Ti
4+
pillared bentonite by means to evaluate the
effect of pH, adsorbent weight, adsorption time,
initial concentration of dyes and temperature. The
kinetics and thermodynamic parameters were also
evaluated and calculated based on dyes remaining
concentration and amount of adsorbed species
measured by spectrophotometer UV-Vis.
Here, we report the change of character between
natural bentonite and Fe
3+
/Ti
4+
pillared bentonite
(made in 2:1 and 1:2 ratio). Both materials was used
to adsorb dyes. This work was aimed to determine
functional group changes and to evaluate the
successful of bentonite pillarization concluded from
data obtained from FTIR spectrophotometer and
XR-Diffraction.
Said, M., Antini, R., Taher, T. and Lesbani, A.
Pillarization of Bentonite using Fe3+/Ti4+ and Its Application for Congo Red and Direct Violet Removal.
DOI: 10.5220/0008854400790088
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 79-88
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
79

2 MATERIALS AND METHODS
2.1 Materials
Chemicals used in this research was analytical grade
i.e. hydrochloric acids (HCl), sulphuric acids
(H
2
SO
4
), iron (III) nitrate (Fe(NO
3
)
3
, sodium
hydroxide (NaOH), sodium carbonate (Na
2
CO
3
),
sodium chloride (NaCl), titanium (IV) isopropoxyde
as well as bentonite, congo red and direct violet dyes
and distilled water.
The pillared bentonite was achieved by pillaring
chemicals with bentonite. The mixture was stirred
for 24 hours and then filtered and dried in oven for
the next 24 hours. The pillared bentonite was
calcined for 2 hours at 400
o
C. The product was
characterized by using XRD and FTIR.
2.2 Congo red and direct violet dyes
Adsorption Experiments
Congo red (CR) and direct violet (DV) adsorption
experiments were conducted in batch reactor.
Adsorption was carried out in various pH (1-9),
adsorbent weight (0.01; 0.03; 0.1; 0.2 and 0.3 g),
adsorption time (5, 10, 15, 20, 30, 45 and 60
minutes), CR and DV initial concentration and
temperature (30, 50 and 70
o
C). The adsorption
procedure is as followed, certain amount of Fe/Ti
pillared bentonite was mixed with 50 mL dyes (CR
and DV) at various concentration. The pH of
mixtures was set at particular condition. Horizontal
shaker was used to homogenize adsorption mixtures
for pre-determined period of time. The mixture
finally was filtered and the remaining dyes were
measured by using spectroscopy UV-Visible.
The effect of various parameters was evaluated
against Fe/Ti pillared bentonite performance on CR
and DV removal from aqueous medium. Parameters
to be evaluated as mentioned above were: solution
pH, adsorbent weight, adsorption time, dyes initial
concentration and temperature.
Adsorption isotherm was evaluated according to
two approaches, Langmuir isotherm and Freundlich
isotherm. Langmuir isotherm was calculated by
using the following formula:
(1)
Where: Q
e
= adsorbate amount (mg/g), K
L
=
equilibrium constant (L/g), C
e
= equilibrium
concentration (mg/g), Q
m
= adsorption maximum
capacity (mg/g)
Freundlich isotherm was also calculated to be
compared with the Langmuir which one is best
followed refereeing by correlation coefficient
obtained. The Freundlich was calculated by using
formula as follows:
log Q
e
= log K
f
+ 1/n log C
e
(2)
Where: Q
e
= adsorbate amount (mg/g), K
f
=
equilibrium constant (L/g), C
e
= equilibrium
concentration (mg/g)
Adsorption rate was assessed according to
adsorption kinetic data which was presumed to
follows one of two approaches used namely pseudo
first order and pseudo second order rate. The
equations used in these approaches were:
(3)
(4)
Where: Q
e
= adsorbate capacity (amg/g), Q
t
=
adsorbate capacity at t (mg/g), t = adsorption time
(minute), k
1
= adsorption rate constant pseudo 1
st
, k
2
= adsorption rate constant pseudo 2
nd
Thermodynamic assessment was carried out
based on the following equation:
(5)
The change of Gibbs free energy, enthalpy as
well as entropy was calculated according to general
equation:
∆G = ∆H - T∆S (6)
Where: Q
e
/C
e
= adsorbate distribution coefficient, R
= gas constant
3 RESULTS AND DISCUSSION
3.1 Natural Bentonite and Fe
3+
/Ti
4+
Pillared Bentonite Characterization
Result from X-Ray Diffraction
(XRD)
Diffraction pattern of natural bentonite and Fe
3+
/Ti
4+
pillared bentonite made with different ratio (2:1 and
1:2) is shown on Figure 1. The increase of basal
spacing due to pillarization process can be
apprehend at 2θ angel 3-10º (Bouraie et al., 2017).
Natural bentonite shows characteristic pattern at
6.39, 19.77, 26.68, and 34.96°. The 2θ at 6.3°
indicates (001) having interlayer distance 13.8 Å.
The exchangeable cations and other ions filled the
space between layers of bentonite (Wang et al,
2016).
Figure 1b represents Fe
3+
/Ti
4+
pillared bentonite
(made with 2:1 ratio) diffractogram reveals
characteristic peaks at 2θ: 5.90; 19.73; 26.51 and
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
80
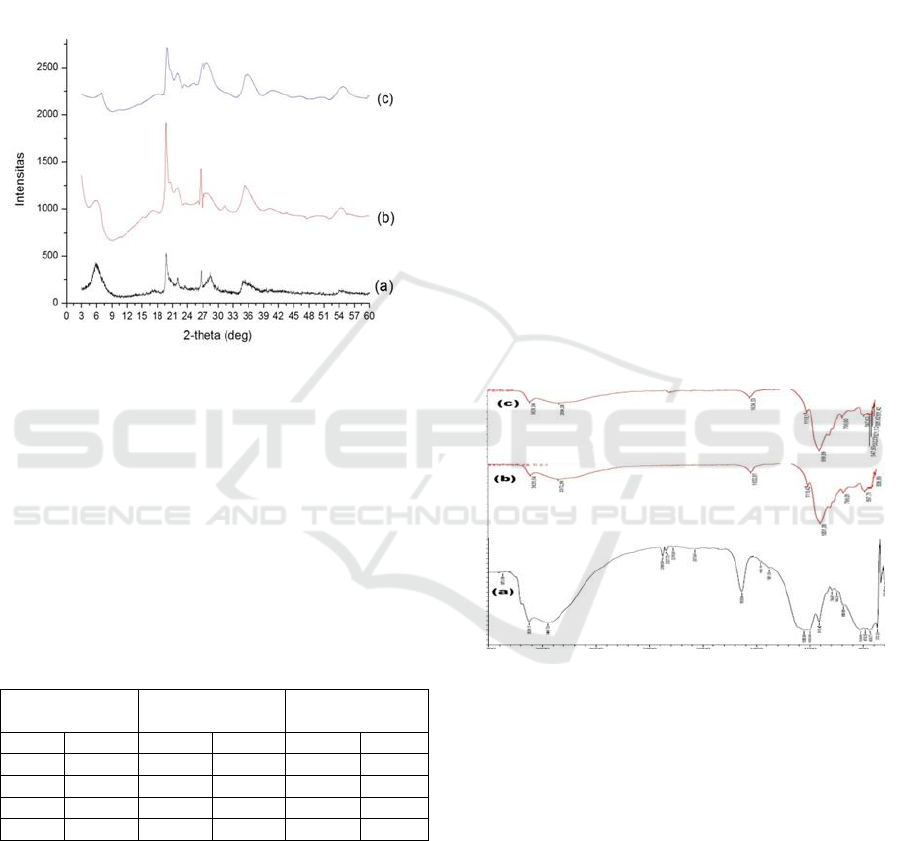
35.04°. The diffraction angel shift at 5.90° resulted
in distance enlargement at (001) up to 15Å. The
Fe3
+
/Ti
4+
pillared bentonite (1:2 ratio) shows
characteristic peaks at 6.87; 19.97; 26.74 and 35.04°
indicates basal spacing width of (001) by 12.86 Å.
XRD pattern confirmed that natural bentonite has
13.8 Å basal spacing which by pillarization at 2:1
increased to 15 Å.
Figure 1: Diffractogram pattern of (a) natural bentonite (b)
Fe
3+
/Ti
4+
pillared bentonite made with 2:1 and
(c) 1:2 ratio.
Bentonite pillared by Fe
3+
/Ti
4+
at 1:2 ratio on
contrary decreased its basal spacing to 12.86 Å. The
change of basal spacing can be seen from peak
shifting to lower angle. Cations of Fe
3+
and Ti
4+
entered into interlayer space of bentonite possibly
were responsible for the interlayer distance changes
(Bouraie et al, 2017).
Table 1: Basal spacing of natural bentonite and Fe
3+
/Ti
4+
pillared bentonite as recorded by XRD
Natural
Bentonite
Fe
3+
/Ti
4+
Pillared
Bentonite 2:1
Fe
3+
/Ti
4+
Pillared
Bentonite 1:2
2θ
d (Å)
2θ
d (Å)
2θ
d (Å)
6.39
13.82
5.90
15
6.87
12.86
19.77
4.48
19.73
4.49
19.97
4.45
26.68
3.33
26.51
3.36
26.74
3.33
34.96
2.56
35.04
2.55
35.04
2.56
Based on XRD diffractogram as summarized on
Table 1, optimal pillarization was achieved by 2:1
ratio of Fe
3+
/Ti
4+
cations. The 2:1 ratio Fe
3+
/Ti
4+
pillared bentonite was chosen to be applied on
removal of dyes combination of Congo red and
direct violet.
3.2 Identification of Dyes Adsorption
by Natural and Pillared Bentonite
using FT-IR Spectrophotometer
Synthetic dyes has functional groups in its molecular
structure which makes them can be identified when
it adsorbed onto bentonite by using FTIR
spectrophotometer. Figure 2 represents the FTIR
spectra for pillared bentonite before and after dyes
uptake.
Figure 2a shows typical absorption of natural
bentonite at wavenumber 3626 and 910 cm
-1
which
represent Al-O-(OH)-Al stretching and bending at
bentonite octahedral layer. Water molecules
presence within bentonite interlayer appears at 3448
and 1635 cm
-1
as H-O-H stretching and bending
vibration.
Strong absorption band at 1033 cm
-1
represents
stretching vibration of Si-O-Si in bentonite
tetrahedral layer whereas bending vibration appears
at 470 cm
-1
. Al-O-Si bending shows at wavenumber
532 whilst at 686 cm
-1
belongs to vibration from
feldspar bonding which associated with bentonite
(Perelomov, 2016).
Figure 2: FT-IR spectra: (a) natural bentonite, (b)
Fe3+/Ti4+ pillared bentonite at 2:1 before and
(c) Fe3+/Ti4+ pillared bentonite at 2:1 after
congo red adsorption.
Fe
3+
/Ti
4+
pillared bentonite FTIR spectra before
adsorption depicted at Figure 2b. Absorption band at
3626 cm
-1
represents stretching vibration of Al-O-
(OH)-Al at bentonite octahedral layer. Bending
vibration of H-O-H detected at wavenumber 1632
cm
-1
whereas stretching vibration of Si-O-Si from
tetrahedral layer of bentonite appears at 1001 cm
-1
.
Stretching vibration of Al-O and Si-O appears at 796
and 508 cm
-1
respectively.
FTIR spectra indicates Fe
3+
/Ti
4+
pillared
bentonite after being used for adsorption shows no
new peaks from dyes being adsorbed nor peaks
change due to adsorption process. The spectra
Pillarization of Bentonite using Fe3+/Ti4+ and Its Application for Congo Red and Direct Violet Removal
81
therefore cannot confirm dyes uptake by pillared
bentonite. Figure 2c however still reveals the
existence of stretching vibration of Si-O-Si at 999
cm
-1
and bending vibration of Si-O at 501 cm-1. The
dyes adsorbing pillared bentonite particularly Congo
red shows peaks at 505-549 cm
-1
as represent of
bending vibration of Al-O-Si.
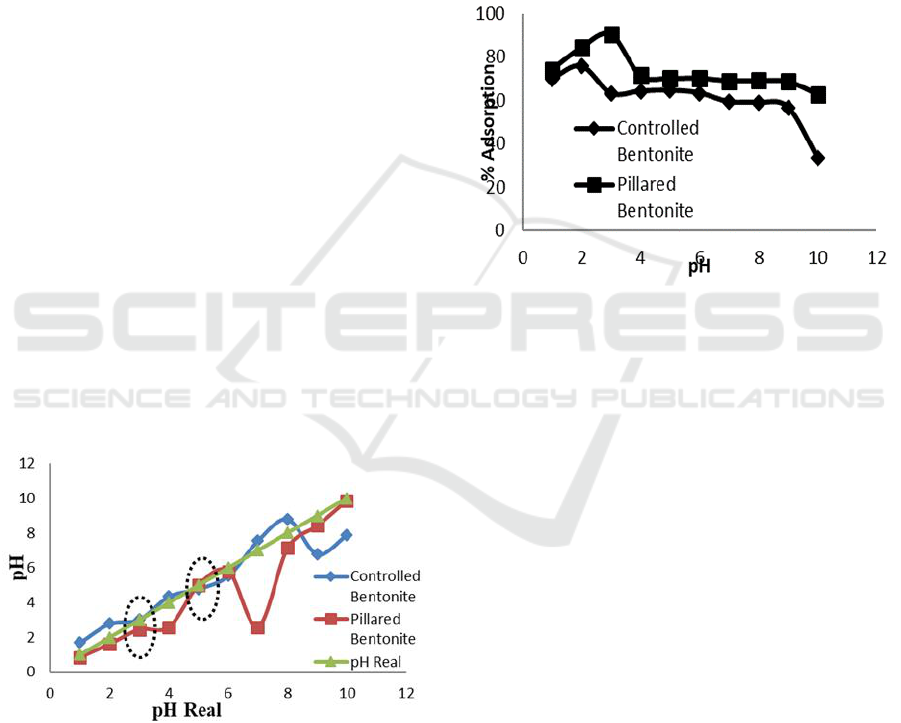
3.3 Point of Zero Charge (PZC)
Analysis
The pH
pzc
was measured to determine charge
property of adsorbent surface i.e. bentonite. This
parameter expresses a condition where at certain pH
the bentonite surface has no charges. Fabryanty et al,
(2007) stated that bentonite will has positive charge
at pH < pH
pzc
and negative charge at pH > pH
pzc
.
The analysis result is shown at Figure 3.
Natural bentonite as shown on Figure 3 has pH
pzc
at pH 3 whilst Fe
3+
/Ti
4+
pillared bentonite at pH 5.
The pH
pzc
value was difference between adsorbents
caused by acidity difference on its surface
(Febryanty et al., 2017). Sahara (2010) explained
bentonite is an anionic clay or layered materials
possesses negative surface charge, hence at pH
below its pH
pzc
the bentonite surface will possesses
positive charge due to excess of H
+
ions. At pH
above pH
pzc
, The bentonite surface will possesses
negative charge caused by excess OH
-
ions presence
at it surface. The charge type of bentonite surface
affects the adsorption capacity of dyes particularly
with opposite charge.
Figure 3: Point zero charge (PZC) of prepared adsorbents
3.4 The Adsorption of Congo red and
Direct Violet by using Natural
Bentonite and Fe
3+
/Ti
4+
Pillared
Bentonite
3.4.1 The Effect of pH
The effect of pH on Congo red and direct violet
adsorption were evaluated at pH 1-10. The
remaining dye was determined by using
Spectrophotometer UV-Vis at λ
max
497 nm. The
result can be seen at Figure 4 and 5.
Figure 4: The effect of pH on congo red adsorption by
using natural bentonite Fe
3+
/Ti
4+
pillared
bentonite.
Figure 4 appears that Fe
3+
/Ti
4+
pillared bentonite
adsorbed more than natural bentonite (control). The
pillared bentonite provides 74-91% adsorption at pH
1-3 but decrease at pH 4-10. Congo red is an anionic
dye which is optimally being adsorbed when the
adsorbent surface has positive charge (low pH). As
the pH becomes more alkaline (pH 4-10). The
bentonite surface start to negatively charged due to
the excess presence of OH- ions. At this point,
anionic dyes such as Congo red face repulsion from
the bentonite surface hence the adsorption number is
decreased. This result was supported by the pH
pzc
value 5 i.e. below this point, Congo red has positive
charge which optimally adsorbed this anionic type
dyes.
The remaining direct violet after being adsorbed
by bentonite was measured by using
spectrophotometer UV-Vis at λmax 546 nm. The
analysis result is displayed at Figure 5.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
82
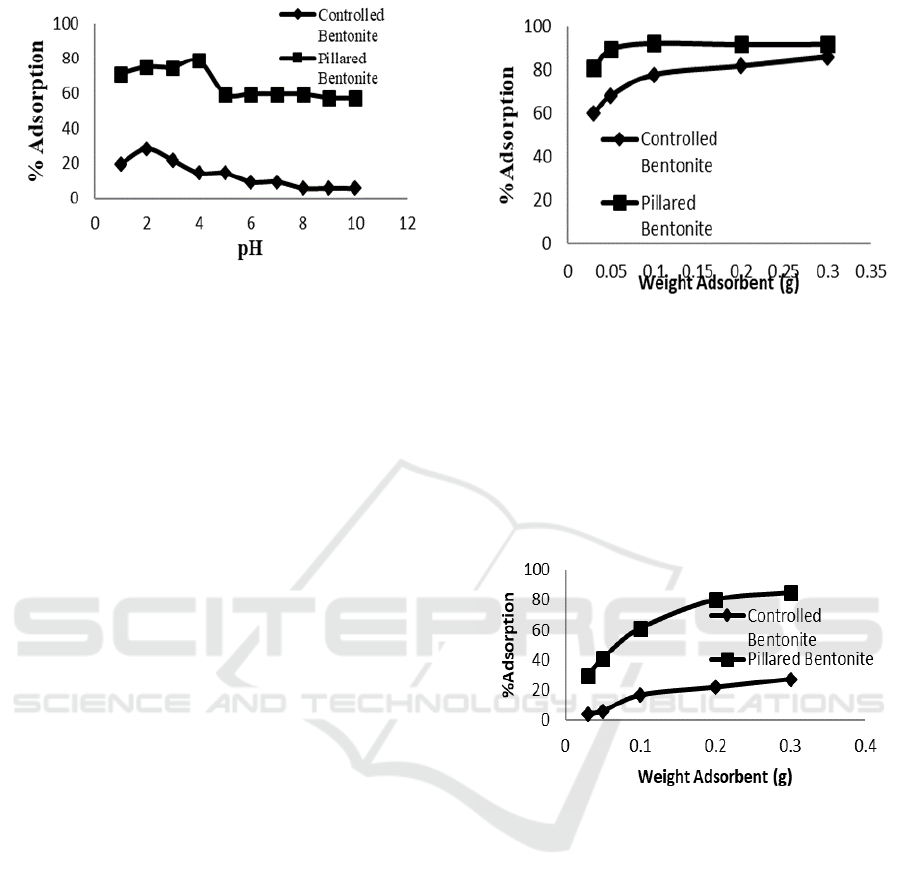
Figure 5: The effect of pH on direct violet adsorption
using natural bentonite and Fe
3+
/Ti
4+
pillared
bentonite.
The direct violet uptake by Fe
3+
/Ti
4+
pillared
bentonite markedly increased at pH 4 by 78%.
Above this optimum pH (5-10), the adsorption tends
to stable or decrease. Direct violet has similar charge
property i.e. anionic dyes hence it shows similar
adsorption trends as solution pH changes. Acidic
condition makes direct violet more being adsorbed
while basic condition less adsorption.
Fe
3+
/Ti
4+
pillared bentonite has pH
pzc
5,
henceforth the anionic direct violet optimally being
adsorbed below this value as the result shown. The
positively charged of bentonite surface at this pH
able to adsorb more direct violet dyes compare to pH
above this value.
3.4.2 The Effect of Adsorbent Weight
The adsorbent weight was varied by 0.03; 0.05; 0.1;
0.2 and 0.3 g at constant concentration of dyes i.e.
100 mg/L. The amount of dyes adsorbed is shown at
Figure 6 and 7.
Figure 6 reveals the adsorption of Congo red
over natural bentonite and Fe
3+
/Ti
4+
pillared
bentonite has similar curve trends but slightly
different amount/percentage. The Fe
3+
/Ti
4+
pillared
bentonite shows remarkable adsorption to achieve
almost 100% Congo red dyes.
Figure 6: The effect of adsorbent weight on Congo red
adsorption
Natural bentonite with original property of its
raw material provides 80% Congo red dyes uptake.
No significant increase of adsorption at adsorbent
weight 0.1 to 0.3 g. It seems that optimum
adsorption was achieved at certain ratio of adsorbent
to adsorbate rather than to maximize the amount of
adsorbent used.
Figure 7: The effect of adsorbent weight on direct violet
adsorption over natural and pillared bentonite
Fe
3+
/Ti
4+
pillared bentonite show huge difference
of % adsorption compare to natural bentonite when
direct violet used as adsorbate. The adsorption
shows similar tendency i.e. increase amount of
adsorbent caused increase amount of adsorption. The
curve slope however indicates the adsorption
increment on natural bentonite is low whereas
pillared bentonite has steeper slope. It can be
concluded that pillared bentonite at the same weight
noticeably adsorbed more direct violet than natural
bentonite.
The maximum amount of direct violet was
adsorbed by pillared bentonite at 0.3 g weight of
adsorbent approximately 80%. This result compares
to Congo red shows lower % adsorption. The
probable cause for this is the structure difference
Pillarization of Bentonite using Fe3+/Ti4+ and Its Application for Congo Red and Direct Violet Removal
83
between both anionic dyes. Congo red has simpler
structure with lower number of aromatic molecule
whereas direct violet has more aromatic molecules
on its molecular structure.
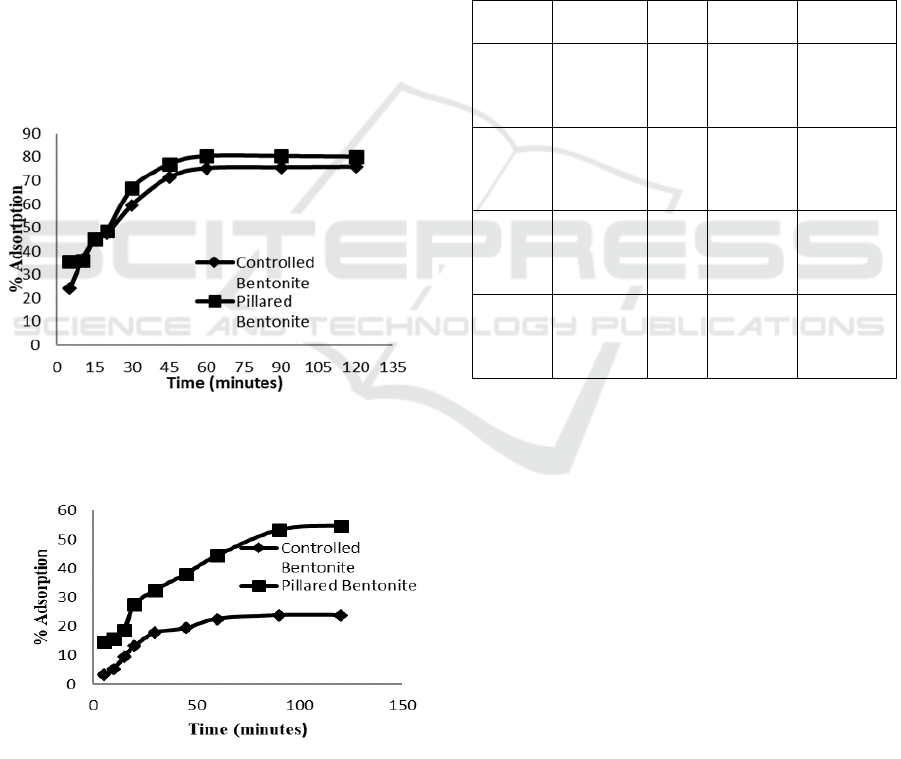
3.4.3 The Effect of Adsorption Time and Its
Kinetic Overview
Adsorption of solute adsorbate needs an optimum
time to obtain maximum result. Result of adsorption
of Congo red and direct violet at various adsorption
time is shown on Figure 8 and 9.
Fe
3+
/Ti
4+
pillared bentonite shows its superiority
over natural bentonite in both dye adsorption. At the
same adsorption time, pillared bentonite provides
more dyes uptake than the natural one. The
performance of pillared bentonite shows different
way between the two dyes adsorbate. Adsorption
equilibrium of Congo red over pillared bentonite
was achieved at 60 minutes whereas direct violet
needs 90 minute to achieve equilibrium.
Figure 8: The effect of adsorption time on Congo red
removal over natural bentonite and Fe
3+
/Ti
4+
pillared bentonite
Figure 9: The effect of adsorption time on direct violet
removal over natural bentonite and Fe3+/Ti4+
pillared bentonite
Maximum amount of dyes uptake by using
pillared bentonite at equilibrium condition is 75-
80% for Congo red while direct violet is being
adsorbed by 23-53%. No further adsorption occurs
after equilibrium due to adsorbent surface is
adsorbing at the same rate with desorption process
(Bentahar et al, 2017).
Adsorption rate of dyes over natural and pillared
bentonite was calculated using time variable of
adsorption. The pseudo-first order as well as the
pseudo-second order were used as model approach.
Several parameters were obtained by this calculation
as shown on Table 2.
Table 2: Kinetics model constants of Congo red and direct
violet adsorption over natural and pillared bentonite
Kinetic
model
Parameter
Dyes
type
Control
bentonite
Pillared
bentonite
Pseudo-
first-
order
Qe exp
Qe
K
1
R
2
CR
75.7602
77.0785
0.0591
0.9656
80.1301
88.6103
0.0686
0.9367
Pesudo-
second-
order
Qe exp
Qe
K
1
R
2
CR
75.7602
85.8321
0.00091
0.9956
80.1301
90.3501
0.0008
0.9887
Pseudo-
first-
order
Qe exp
Qe
K
1
R
2
DV
23.8676
54.2814
0.0784
0.9020
54.7647
60.7603
0.0351
0.9034
Pesudo-
second-
order
Qe exp
Qe
K
1
R
2
DV
23.8676
62.8931
0.00022
0.94714
54.7647
68.6696
0.00045
0.9724
Table 2 informs the calculation result based on
pseudo-second order approach gave correlation
coefficients greater than pseudo-first order for both
Congo red and direct violet adsorbate on natural and
pillared bentonite. Correlation coefficients of
pseudo-second order for Congo red is 0.9956 over
natural bentonite and 0.9887 over Fe
3+
/Ti
4+
pillared
bentonite. Direct violet shows correlation
coefficients 0.9484 over natural bentonite and
0.97014 over Fe
3+
/Ti
4+
pillared bentonite.
These results suggest that the adsorption process
is not first order. Pseudo-second order governed
according to assumption that rate limiting step of the
adsorption might be chemical interaction between
adsorbate and adsorbent. This interaction could
involve valence forces through electron exchange
between anionic molecules and charged surface of
bentonite.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
84
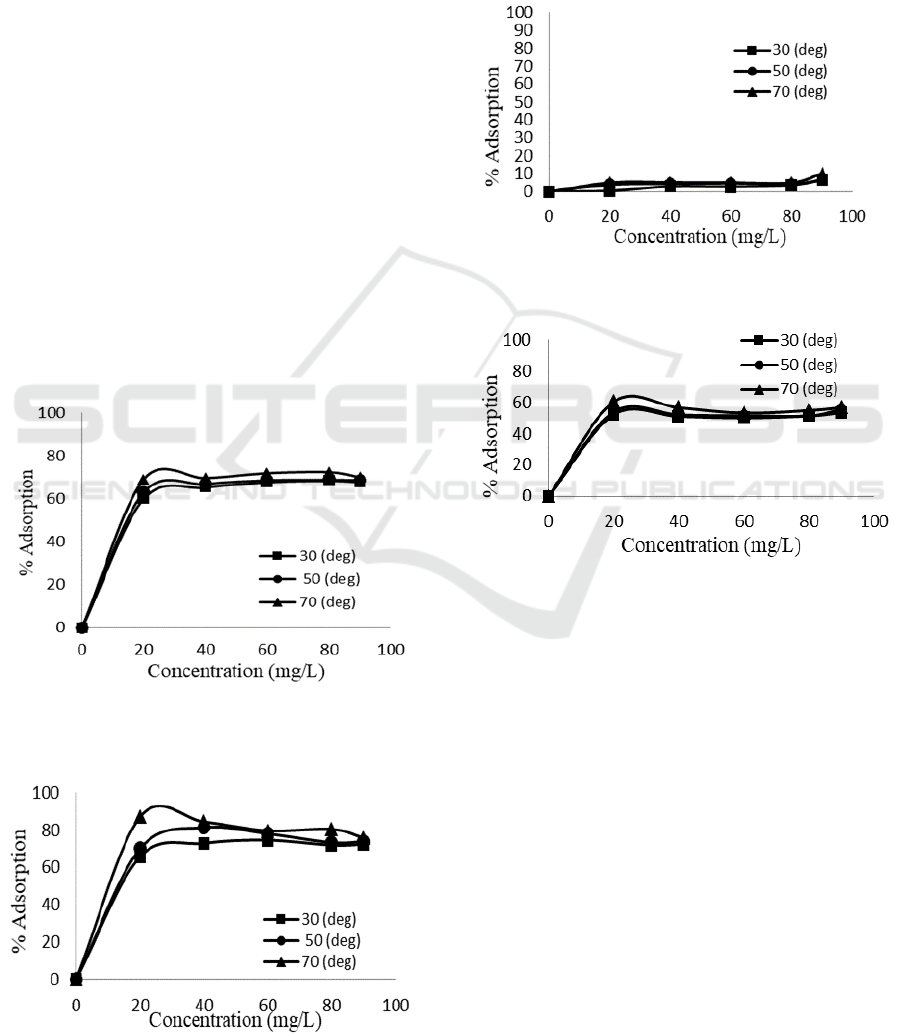
3.4.4 The Effect of Concentration and
Temperature and Its Thermodynamic
Overview
Adsorption of Congo red onto natural bentonite and
pillared bentonite was conducted at various
concentration and temperatures at pH 3 in a
horizontal shaker for 60 minutes. Remaining dyes
was measured by using spectrophotometer UV-Vis
at λ
max
497 nm. The measurement which then was
calculated with calibration curve is shown at Figure
10 and 11.
Figure 10 and 11 noticeably point out the similar
trend for both adsorbents that is at higher
concentration and temperature the amount of dyes
adsorbed also raised. The adsorption equilibrium in
general was achieved at concentration above 40
mg/L for all temperature in which adsorption
conducted. Pillared bentonite although shows similar
equilibrium concentration, it has slightly higher
adsorption amount compare to natural bentonite.
Higher concentration means more frequent collision
between molecules of adsorbate and adsorbent
whereas higher temperature provides a bigger
fraction of molecules with enough energy to
performed adsorption process.
Figure 10: The effect of concentration and temperature
on Congo red adsorption over natural
bentonite
Figure 11: The effect of concentration and temperature on
Congo red adsorption over Fe
3+
/Ti
4+
pillared
bentonite
Direct violet adsorption was conducted at pH 4
and same duration (60 minutes) in a horizontal
shaker. The adsorption process for this adsorbate
was measured and calculated as shown on Figure 12
and 13.
Figure 12: The effect of concentration and temperature on
direct violet adsorption onto natural bentonite
Figure 13: The effect of concentration and temperature on
direct violet adsorption onto Fe
3+
/Ti
4+
pillared
bentonite
The result of concentration and temperature
effect on direct violet adsorption is similar to what
was found on Congo red. The difference between
natural bentonite and pillared bentonite however, is
remarkably high. The increase of adsorption along
with concentration and temperature increase on
pillared bentonite dwarf the increment on natural
bentonite. Equilibrium condition of direct violet
adsorption over pillared bentonite was achieved at
60% while natural bentonite can only obtained less
than 10%.
At particular temperature, the amount of
adsorption reveals an adsorption isotherm. Two
models were used in this report i.e. Langmuir and
Freundlich. Langmuir isotherm assumed that the
adsorption occurred in monolayer fashion provided
Pillarization of Bentonite using Fe3+/Ti4+ and Its Application for Congo Red and Direct Violet Removal
85
that the surface is homogeny. Freundlich isotherm
on contrary is a special case of Langmuir approach.
According to this theory, the adsorption occurs in a
multilayer mode on a heterogeneous surface. The
difference between these two models also involved
interaction types between adsorbate and adsorbent
i.e. chemical or physical. Table 3 and 4 show
calculation result for both models along with several
constants as stated in its formula.
Table 3: Isotherm data calculated according to Freundlich
model
Temp
(
o
C)
Parameter
Dyes
Control
bentonite
Pillared
bentonite
30
K
f
N
R
2
CR
1.986
0.783
0.995
5.993
0.838
0.964
50
K
f
N
R
2
1.208
0.841
0.997
3.603
1.063
0.848
70
K
f
N
R
2
1.520
1.000
0.931
9.687
1.520
0.985
30
K
f
N
R
2
DV
4.276
0.373
0.984
1.054
0.997
0.993
50
K
f
N
R
2
0.019
0.810
0.957
1.152
1.010
0.986
70
K
f
N
R
2
0.002
0.790
0.863
1.150
1.950
0.986
Table 4: Isotherm data calculated according to Langmuir
model
Temp
(
o
C)
Parameter
Dyes
Control
bentonite
Pillared
bentonite
30
K
L
Q
m
R
2
CR
0.013
114.9
0.761
0.010
204.0
0.285
50
K
L
Q
m
R
2
0.009
175.4
0.746
0.005
614.9
0.027
70
K
L
Q
m
R
2
0.005
208.3
0.075
0.064
119.0
0.947
30
K
L
Q
m
R
2
DV
0.011
0.374
0.632
0.017
12.75
0.786
50
K
L
Q
m
R
2
0.004
9.442
0.522
0.018
14.02
0.745
70
K
L
Q
m
R
2
0.001
3.172
0.699
0.013
33.22
0.890
The effect of temperature on dyes adsorption was
tabulated in Table 5 and 6. Based on these data,
several thermodynamics parameters were calculated
i.e. Gibbs free energy (∆G), enthalpy (∆H) and
entropy (∆S). The calculation of ∆H and ∆S was
carried out by using equation 6 of 1/T versus ln
qe/ce particularly from its slope and intercept. The
value of ∆G was calculated by using equation 7
based on ∆H and ∆S obtained.
Table 5 and 6 displays result of thermodynamic
calculation for Congo red adsorption over natural
and pillared bentonite. Adsorption capacity (qe) of
both natural bentonite and pillared bentonite was
increase proportionally as temperature of adsorption
raised.
Table 5: Adsorption energy (E, kJ/mol), entropy (∆S,
kJ/mol), enthalpy (∆H, kJ/mol) and adsorption
capacity (qe) of Congo red adsorption over
natural bentonite at various temperature
Conc.
(mg/L)
T
(K)
Q
e
∆S
∆H
E
20
303
323
343
12.01
12.62
13.80
30.96
8.413
0.96
1.58
2.17
40
303
323
343
26.10
27.78
40.60
18.78
4.124
1.56
1.99
2.29
60
302
323
343
40.60
41.18
43.07
19.52
4.093
1.82
2.21
2.58
80
303
323
343
54.70
55.08
57.80
19.23
3.94
1.88
2.26
2.67
90
303
323
343
61.02
61.74
62.89
12.99
2.071
1.86
2.12
2.37
Table 6: Adsorption energy (E, kJ/mol), entropy (∆S,
kJ/mol), enthalpy (∆H, kJ/mol) and adsorption
capacity (qe) of Congo red adsorption over
Fe
3+
/Ti
4+
pillared bentonite at various
temperature
Conc.
(mg/L)
T
(K)
Q
e
∆S
∆H
E
20
303
323
343
13.12
14.01
17.43
93.34
27.02
1.26
3.12
4.90
40
303
323
343
29.12
32.57
33.80
96.11
15.48
2.57
3.76
4.89
60
302
323
343
44.67
46.88
47.84
30.58
6.537
2.79
3.34
3.92
80
303
323
343
57.44
58.70
64.45
41.52
10.26
2.21
3.04
3.83
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
86
90
303
323
343
64.98
66.45
68.45
22.17
4.332
2.38
2.82
3.25
The adsorption capacity of pillared bentonite
shows greater value at high concentration of Congo
red for all temperature measured. Pillarization
treatment was able to increase basal spacing between
layers of bentonite hence the capacity of pillared
bentonite to adsorb is bigger.
Result of energy calculation exposes that the
adsorption energy is positive and increase as
temperature rise. Normally, adsorption is an
exothermic event hence has negative energy. The
unbalance energy at surface was compensated by
adsorbing solid adsorbate so it decreases its energy.
The situation for adsorption in solution however
must take into account solvent contribution.
Fontecha-Ca´mara et al. (2006) suspected that the
interaction of solvent-adsorbent and solvent-
adsorbate could shifts the exothermic mode of
adsorption into endothermic one.
The calculated parameters of thermodynamic
based on adsorption of direct violet at various
temperatures is displayed on Table 7. The adsorption
was conducted over Fe
3+
/Ti
4+
pillared bentonite.
Table 7: Adsorption energy (E), entropy (∆S), enthalpy
(∆H) and adsorption capacity (qe) of direct
violet adsorption over Fe3+/Ti4+ pillared
bentonite at various temperature
Conc.
(mg/L)
T
(K)
Q
e
∆S
∆H
E
20
303
323
343
10.42
10.74
12.10
24.51
7.306
0.12
0.61
1.10
40
303
323
343
20.37
20.73
23.70
16.54
4.988
0.02
0.35
0.68
60
302
323
343
30.00
30.78
31.98
9.51
2.847
0.06
0.27
0.49
80
303
323
343
40.98
41.00
43.38
10.71
3.183
0.06
0.27
0.49
90
303
323
343
47.98
49.80
51.08
11.10
3.023
0.34
0.56
0.78
The Gibbs free energy (∆G) for both Congo red and
direct violet according to calculation result is
negative which means it occurs spontaneously.
Table 7 shows similar trends with previous table.
Entropy value obtained nonetheless shows large
difference. As temperature increase, so does entropy
of both adsorptions. Direct violet appears slightly
smaller entropy compare to Congo red. The
complexity of adsorbate structure might hinder the
adsorption process due to steric hindrance so it
lowers entropy of the process. Enthalpy result is
quite similar as Congo red. The type of interaction
for adsorption in solution possess was difficulty and
not as simple as gas adsorption over solid adsorbent.
4 CONCLUSIONS
Basal spacing of natural bentonite was successfully
increased from 13.8 to 15 Å through pillarization
using Fe
3+
/Ti
4+
at 2:1 ratio. Quantitatively,
spectrophotometer UV-Vis confirmed the dyes
removal from decrease of intensity but FTIR spectra
gave no peaks indication of the functional groups of
corresponding dyes. The optimum adsorption
condition is in accordance with pH
pzc
i.e. higher at
pH below pH
pzc
. Freundlich model for heterogenic
surface along with possible chemical interaction is
best fit to the process conducted. Thermodynamic of
adsorption suggest the process is endothermic and
occurred spontaneously.
ACKNOWLEDGEMENTS
The first author want to say thank you to
KEMENRISTEKDIKTI for the financial support
through Hibah Penelitian Dasar 2019.
REFERENCES
Bentahar, S., Abdellah, D., Mohammed, E.L., and
Noureddine, E.M., 2017. Adsorption of Methylene
Blue, Crystal Violet and Congo red From Binary and
Ternary Systems with Natural Clay: Kinetic, Isotherm,
and Thermodynamic. Journal of Environmental
Chemical Engineering. 5: 5921-5932.
Chinoune, K., Bentaleb, K., Bouberka, Z., Nadim, A., and
Maschke, U., 2016. Adsorpsi of Reactive Dyes from
Aqueous Solution by Dirty Bentonite. Applied Clay
Science. 132: 64-75.
Cool, P., and Vansant, E.F., 1998. Pillared Clays:
Preparation, Characterization and Applications.
University of Antwerp (UIA): Laboratory of Inorganic
Chemistry, Department of Chemistry.
Fabryanty, R., Valencia, C., Soetaredjo, F.E., Putro, J.N.,
Santoso, S.P., Kurniawan, A., Ju, Y.H., and Ismadji,
S., 2017. Removal of Crystal Violet Dye by
Adsorption Using Bentonite – Alginate Composite.
Pillarization of Bentonite using Fe3+/Ti4+ and Its Application for Congo Red and Direct Violet Removal
87

Journal of Environmental Chemical Engineering. 22:
3-37.
Fontecha-Cámara, M. A., López-Ramón, M. V., Álvarez-
Merino, M. A., & Moreno-Castilla, C., 2006. About
the endothermic nature of the adsorption of the
herbicide diuron from aqueous solutions on activated
carbon fiber. Carbon, 44(11), 2335–2338.
Georgescu, A.M., Nardou, F., Valentin, Z., and Nistor,
I.D., 2017. Adsorption of Lead (II) Ions from Aqueous
Solution onto Cr-Pillared Clay. Applied Clay Science.
21: 15-22.
Goodarzi, A, R., Najafi, F, S., and Shekary, H., 2016.
Impact of Organic Pollutants on the Macro and Micro
Structure Responses of Na-Bentonite. Applied Clay
Science. 121: 17-28.
Hao, Y., Yan, L., Yu, H., Yang, K., Yu, S., Shan, R., and
Du, B., 2014. Comparative Study on Adsorption of
Basic and Acid Dyes by Hidroxy-Alumunium Pillared
Bentonite. Journal of Molecular Liquids. 199: 202-
207.
Hou, M., Ma, C., Zhang, W., Tang, X., Fan, Y., and Wan,
H., 2011. Removal of Rhodamine B using Iron-
Pillared Bentonite. Journal of Hazardous Materials.
186: 1118-1123.
Kumararaja, P., Manjaiah, K.M., Datta, S.C., and Binoy,
S., 2017. Remediation of Metal Contaminated Soil by
Alumunium Pillared Bentonite: Synthesis,
Characterisation, Equilibrium Study and Plant Growth
Experiment. Applied Clay Science. 137: 115-122.
Kaufhold, S., and Dohromann, R., 2008. Detachment of
Colloidal Particles From Bentonites in Water.
Aplplied Clay Science. 39: 50-59.
Martin, Y., Permata, D., Despa, D., and Wiyoto, Y.L.,
2019. The Use of Physically Activated and Soil
Composed Bentonite as Enviroment Friendly for
Grounding Resistance. The International Conference
Reasearch Collaboration of Enviromental Science.
245: 1-9.
Okoye, I.P and Obi, C., 2011. Synthesis and
Characterization of Titanium Pillared Bentonite Clay
Mineral. International Archieve of Applied Sciences
and Technology. 2: 84-89.
Tomul, F., 2016. The Effect of Ultrasonic Treatment on
Iron-Chromium Pillared Bentonites Synthesis and
Catalytic Wet Peroxide Oxidation of Phenol. Applied
Clay Science. 120: 121-134.
Unuabonah, E, I., Adebowale, K, O., and Dawodu, F, A.,
2008. Equilibrium, Kinetic and Sorber Design Studies
on the Adsorption of Aniline Blue Dye by Sodium
Tetraborate-Modified Kaolinite Clay Adsorbent.
Journal of Hazardous Materials. 157: 397-409.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
88
