
Synthesis of Carboxymethyl Polysaccharide from Arenga Pinnata
Polysaccharide and Monochloroasetic
Juliati Br. Tarigan
1*
, Diana A. Barus
2
and Nico Hot Asi Naibahao
1
1
Department of Chemistry,Universitas Sumatera Utara, Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Department of Physics, Universitas Sumatera Utara, Medan, Indonesia
Keywords: Arenga pinnata Merr., polysaccharide, carboxymethyl, monochloroacetate.
Abstract: Synthesis of carboxymethyl polysaccharide Arenga pinnata has been prepared through etherification of
Arenga pinnata polysaccharide and monochloroacetate (MCA). Firstly the polysaccharide was extracted
from Arenga pinnata endosperm with different toughness (soft and hard) followed by etherification with
monochloroacetate using sodium hydroxide as a catalyst with variation of molar ratio MCA : NaOH of 0.50;
0.55; and 0.60 and ethanol 96% as solvent at temperature of 60C for 10 hours in oven. The substitution
degree was determined using titration method in which the Na-carboxymethyl was hydrolysis with HCl 2M
in methanol 70% to form carboxymethyl polysaccharide. The yields of carboxymethyl polysaccharide were
90% and 83% for hard and soft, respectively. The substitution degree of carboxymethyl polysaccharide
from soft Arenga pinnata endosperm was 1.600; 1.068 and 0.403 and from hard endosperm was 0.688;
0.467 and 0.202. The substitution degree of carboxymethyl polysaccharide was increased in the increasing
of NaOH used which polysaccharide from Arenga pinnata endosperm soft has higher substitution degree
than from the hard. The appearance stretching vibration of the carbonyl group (C=O) at a wavelength of
1730 cm-1 confirmed the formation of carboxymethyl polysaccharide. The morphology surface of
carboxymethyl polysaccharide showed rough surface than Arenga pinnata polysaccharide revealed that the
granule of polysaccharide have been changed.
1 INTRODUCTION
Chemical modification of polysaccharides is
considered as one very important pathway to
improve the properties of this biopolymer. Recently
research has been directed towards the
functionalization of a material. Carboxymethylation
of polysaccharides is one of the conversions widely
studied for the development of new biomaterials
with very promising applications (Parvathy et al.,
2005). Carboxymethylation could improve solubility
in water (increases hydrophilicity), insoluble
polysaccharides or low solubility in water (Yang and
Zhang, 2009). Gum polysaccharides are often not
completely dispersed and commercially available
gum solutions are usually cloudy and contain
suspended solid particles. Such solutions are usually
quite thick and cannot be filtered to separate
suspended solids, for this purpose they need to be
modified into carboxymethyl gum which can form
bright dispersion solutions compared to untreated
gum (Moe, 1949). One source of polysaccharide that
is abundant in Indonesia is the endosperm of palm
seeds (Arenga pinnata Merr.) which is often known
as “kolang-kaling” (Mogea et al., 1991). The
utilization of this endosperm is remaining limited for
food (Orwa et al., 2009, Tarigan et al., 2018b).
The polysaccharide containing in Arenga pinnata
endosperm (APE) is galactomannan consisting of
mannose and galactose with a ratio of galactose :
mannose of 1 : 1,33. Those monosaccharide linked
in a linear chain of 1,4- β-D-mannopyranosil and is
water soluble if the galactose residue is >5% and is
insoluble in water if <5% (Aspinall, 1959, Bento et
al., 2013, Tarigan et al., 2012, Tarigan et al., 2018a).
Carboxymethylation reactions have been studied
in some natural polymers such as cellulose,
funegeric gum, sesbania gum flour, guar gum (Gong
et al., 2012, Liyanage et al., 2015, Noleto et al.,
2009, Parvathy et al., 2005). In general, the
carboxymethylation method is carried out using the
catalyst NaOH and NaHCO
3
and monochloroacetate
(MCA) reagents. Reactions can take place either in
with or without water in increasing temperatures
Tarigan, J., Barus, D. and Hot Asi Naibaho, N.
Synthesis of Carboxymethyl Polysaccharide from Arenga Pinnata Polysaccharide and Monochloroasetic.
DOI: 10.5220/0008854100630068
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 63-68
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
63

(Parvathy et al., 2005). Gong et al. (2012) have
examined the synthesis and characterization of
carboxymethyl guar gum with the dry method and
alkalization process using NaOH catalyst and
carboxymethylation using (MCA) and the degree of
substitution was determined by titrimetric. The dry
method is used to avoid the formation of gels that
can resist the effective penetration of the catalyst
and reagent rendering incomplete reaction resulting
in very low substitution degrees.
Based on the description above, this study aimed
to modify the polysaccharide of APE into
carboxymethyl using MCA and NaOH as a catalyst
through the dry method. The type of APE powder
used is hard and soft which is determined by the
penetrometer. The carboxymethyl obtained was
determined by FT-IR spectrophotometer, surface
properties with SEM, and degree of substitution by
titration.
2 MATERIALS AND METHODS
2.1 Materials
The Arenga pinnata was collected from a traditional
market in Medan – Indonesia. All chemicals used in
this study were brought from the local chemical
supplier without any purification.
2.2 Methods
2.2.1 The APE Texture Determination
The stratified random sampling method was used to
collect palm seeds. Texture measurements are
carried out using a penetrometer. The prepared
sample was stabbed at four points using a precision
penetrometer which was pressured 250 g with a
scale of 1/10 mm for 10 seconds. Texture values can
be read on the scale indicated by the needle
instructions and the four values are averaged. The
texture value was calculated with the formula as
below.
/
/
/
(1)
2.2.2 Preparation APE Extract
The preparation of APE extract was conducted
following the previous procedure (Tarigan and
Purba, 2015). The APE group I (hard) was chopped
as thick as 2 mm and crushed roughly with a blender
at a scale speed of 1 for 2 minutes with 250 mL of
water. The hydrogel obtained was immersed in 96%
ethanol for 48 hours at a ratio of 1: 2, then filtered,
the residue obtained was soaked again with ethanol
which was half volume from the initial ethanol
volume for 48 hours and finally with pure grade
ethanol for 12 hours until the residue is submerged.
The residue obtained is dried in a desiccator in a
vacuum until the weight is constant. The same
procedure is conducted for soft palm seeds (group
II). The obtained APE powder was characterized by
FT-IR analysis and SEM surface morphology.
2.2.3 Preparation of Carboxymethyl APE
The preparation of carboxymethyl APE was done
using a method developed by Gong et al. (2012)
with slight modification. 1.65 grams of
galactomannan from APE group I were put into
glass beakers and then added with NaOH in varying
concentration. The mole ratio of MCA : NaOH used
was 1:0.5; 1:0.55 and 1:0.6. Next, the solution was
added with 1 mL ethanol 96%, heated at 30
o
C while
stirring using a magnetic stirrer for 20 minutes and
then was added 1.05 grams of MCA and stirring
using a magnetic stirrer for another 20 minutes, then
transferred into a watch glass and heated in the oven
for 10 hours with a temperature of 60
o
C.
Carboxymethyl polysaccharide produced was dried
at room temperature, crushed and stored in a
desiccator. The same treatment was carried out for
APE group II.
2.2.4 Determination of Substitution Degree
The determination of substitution degree according
to the procedure established by the previous
researcher (Gong et al., 2012). Determination of
substitution degree (SD) was carried out firstly with
converting Na-carboxymethyl polysaccharide into
carboxymethyl polysaccharide of palm seeds. 1.5
grams of Na-carboxymethyl polysaccharide from the
group I with a mole ratio of MCA : NaOH of 1:0.50
was poured into a glass beaker which already
contains 2 M HCl (in methanol 70%), stirred with a
magnetic stirrer for 2 hours, then filtered. The solids
of carboxymethyl polysaccharide obtained were then
washed using ethanol 96% until chlorine free (tested
using silver nitrate / AgNO3 0.1 M solution). The
resulting solid is dried in an oven at 60
o
C for 2
hours. 0.2 grams of carboxymethyl polysaccharide
that has been dried then dissolved in 20 mL of 0.1 M
NaOH, stirred for 2 hours with a magnetic strider.
The carboxymethyl polysaccharide which has excess
NaOH is then titrated using a standard 0.1 M HCl
solution with the addition of phenolphthalein as an
indicator. The same treatment was carried out for the
comparison of MCA : NaOH 0.55; 0.60 and
carboxymethyl galactomannan from APE group II.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
64

The carboxymethyl polysaccharides obtained were
analyzed using FT-IR spectrophotometer and SEM.
3 RESULTS AND DISCUSSIONS
3.1 Texture
The sample is prepared by grouping samples using
the stratified random sampling method. The
population of 100 APE is grouped into 2 groups with
almost the same level of similarity. Group 1 (50
pieces) and group 2 (50 pieces). Samples were taken
randomly at 2% for each group which was for
groups 1 (10 pieces) and group 2 (10 pieces) and
determined in 4 positions namely: up, down, right
and left, then taken evenly the points, but the data is
not included in this paper. The texture and yield of
APE powder and yield carboxymethyl product
showed in Table 1.
Tabel 1: The texture value and yield of APE powder and
carboxymethyl
Parameter
Seed of A. pinata
Hard Soft
Texture
(g/mm)
0.1054–0.1342 0.0846-0.0999
Yield of APE
powder (%)
4.72 4.52
Yield of
carboxymethyl
(%)
90 83
The difference in texture value (Table 1) is
caused by the level of maturity which in soft APE is
lower than that of hard APE. The more mature the
sugar palm seeds, the harder and the higher the
content in the endosperm, the more resistant to
mechanical damage even to water (Cerqueira et al.,
2009). The hard and soft texture is also affected by
the treatment given to the process of making APE,
for example, soaking time, cooking, stripping, etc.
that allow for differences in the texture between one
APE and another. Thus the texture obtained is
different from the texture we have reported in our
previous study, where the hard and soft texture of
the value is lower than our previous research report,
this is because the APE is used uniformly and are
softer and rounder (not flattened) and the source is
also different. Likewise, the percentage of the APE
powder obtained is also different from our previous
research.
3.2 FT-IR Analysis
FT-IR spectrum (Figure 1) shows the peak
characteristics of galactomannan which is similar
with the literature of Boual et al. (2015) and
(Tarigan, 2014); there is no difference in absorption
bands for hard and soft APE, respectively. The
absorption peak at 3345 cm
-1
shows the stretching
vibration of the OH group from the polysaccharide
and the absorption band at 2888 cm
-1
shows the
stretching vibration -CH of -CH
2
- (Singh et al.,
2009) supported by bending vibrations -CH
2
- on the
wavelength of 1372 and 1373 cm
-1
. Absorption at
1638 and 1636 cm
-1
indicates the presence of an OH
group bound to water. The wide peaks at an area of
900 - 1200 cm
-1
due to stretching vibrations of -C-C-
O, C-OH, and C-O-C from the main polymer chain
(Buriti et al., 2014). The peak at 1021 cm
-1
, 1020
cm
-1
shows the C-O bending vibration of the
pyranose ring. The band at 869 cm
-1
characteristics
of the β-D-manopiranose bond present in the
polysaccharide (Buriti et al., 2014) and the band at
811 cm-1 showed the presence of α-D-
galactopiranose bonds (Buriti et al., 2014).
Carboxymethyl polysaccharide from hard and
soft APE was carried out in two stages, namely the
process of adding NaOH as a catalyst (alkalization)
and formed Na-carboxymethyl polysaccharide
which was analyzed by FT-IR (Figures 2) followed
by the addition of MCA (carboxymethylation). The
carboxymethyl polysaccharide of APE formed was
analyzed with an FT-IR spectrophotometer (Figures
3).
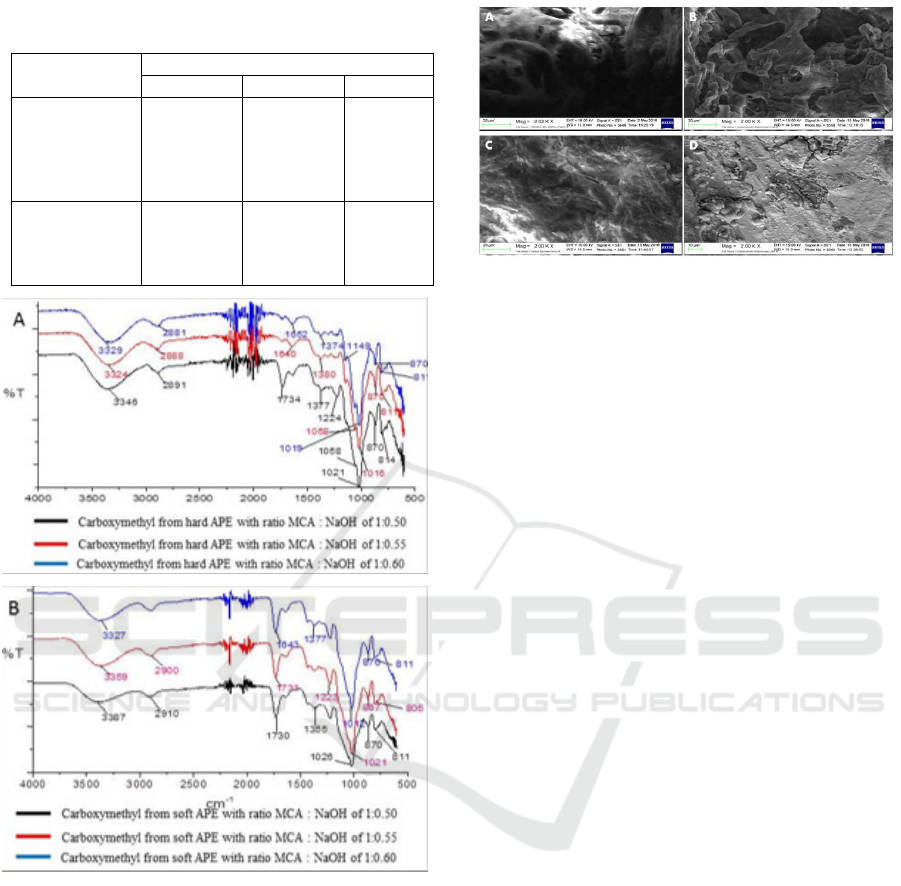
In the formation of Na-Carboxymethyl
polysaccharides the peak changes at wave number
1636 cm
-1
on hard APE and wave number 1638 cm
-1
on soft APE. The formation of carboxymethyl
polysaccharide from hard and soft APE is shown by
the formation of absorption bands at wave numbers
around 1730 cm
-1
which states the formation of
carbonyl groups (C = O). Figure 3A shows that the
peak formation of carboxymethyl carbonyl groups of
hard APE at a ratio MCA : NaOH of 1 : 0.50 while
for carboxymethyl soft palm seeds (figure 3B) the
appearance of carbonyl peaks at mole ratio MCA :
NaOH of 1 : 0.50 and 1 : 0.55. This is inseparable
from the less crystalline polymer structure in soft
APE. The structure is increasingly crystalline
because of the presence of hydrogen bonds
(Niroomand et al., 2016). The release of galactosyl
residues from galactomannan polymers will provide
an increase in the mannose ratio in galactomannan
or the formation of mannan (Bento et al., 2013)
rendering the APE get harder.
Synthesis of Carboxymethyl Polysaccharide from Arenga Pinnata Polysaccharide and Monochloroasetic
65
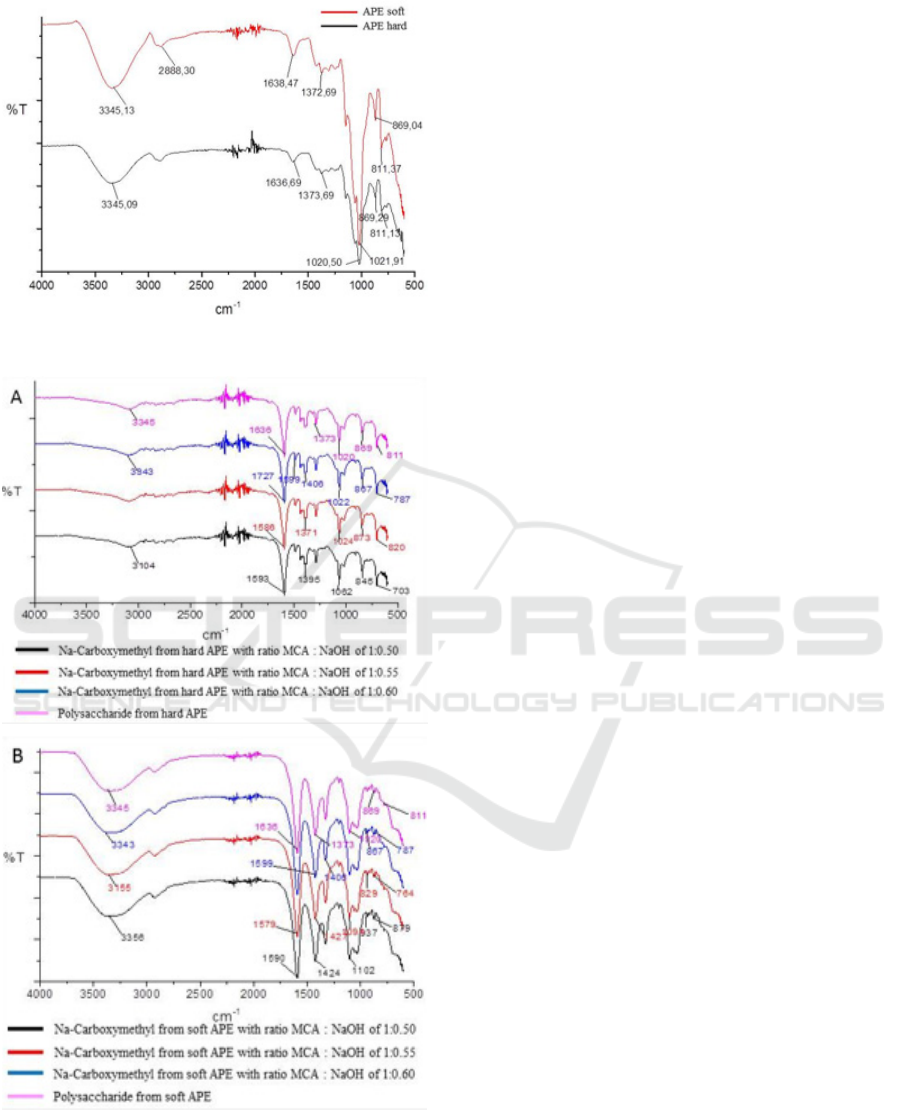
Figure 1: FT-IR spectrum of APE powder hard and soft
Figure 2: FT-IR spectrum of Na-carboxymethyl with ratio
MCA : NaOH of 1 : 0.50; 1 : 0.55; 1 : 0.60 from
(A) hard APE and (B) soft APE.
3.3 SEM Images
The surface morphology images polysaccharide of
hard and soft APE and carboxymethyl
polysaccharide of hard and soft APE is shown in
Figure 4. The surface morphology of hard APE is
indicated by a rough and irregular surface and fused
with each other while soft APE is soft and the
surface is smoother. The morphology of the surface
of carboxymethyl polysaccharides of hard APE
becomes rough and bumpy which indicates that the
interaction between the polymer chains is irregular.
3.4 The Substitution Degree
The substitution degree was determined using
titrimetric method and the result is presented in table
2. The substitution degree (DS) is the average value
of the hydroxyl group exchanging with the
carboxymethyl group present in each
anhydroglucose monomer unit. Substitution degree
test was conducted to determine the number of
carboxyl compounds present in each polysaccharide
monomer. The substitution degree describes the
quality of the carboxymethyl polysaccharide
produced. Theoretically, the maximum substitution
degree value is 3. The substitution degree value
produced from hard APE carboxymethyl
polysaccharide is 0.688 whereas in the
carboxymethyl polysaccharide of soft APE produced
a substitution degree value of 1,600. From the table
above it can be said that the higher or more the
amount of sodium hydroxide (NaOH) is used, the
greater the degree of substitution possessed by the
carboxymethyl polysaccharide. The increasing
amount of NaOH used makes the etherification
process better because the alkali polysaccharide
forms reactivity which is higher in proportion to the
high concentration of NaOH used. The substitution
degree in carboxymethyl polysaccharide of soft APE
is greater than that of carboxymethyl polysaccharide
of hard APE. This is because NaOH is easier to
penetrate increasing swelling properties, for which
monocloroacetic compounds are easier to substitute.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
66
Tabel 2: The substitution degree of carboxymethyl
polysaccharide
Parameter
Mole ratio of MCA : NaOH
0.60 0.55 0.50
SD of
carboxymethyl
polysaccharide
from hard
APE
0.202 0.467 0.688
SD of
carboxymethyl
polysaccharide
from soft APE
0.403 1.068 1.600
Figure 3: The FT-IR spectrum of carboxymethyl
polysaccharide with ratio MCA : NaOH of 1 :
0.50; 1 : 0.55; 1 : 0.60 from (A) hard APE and
(B) soft APE
Figure 4: The SEM images polysaccharide of (A) hard
APE, (B) soft APE and carboxymethyl
polysaccharide from (C) hard APE, (D) soft
APE
4 CONCLUSIONS
Carboxymethyl polysaccharide from hard and soft
APE can be synthesized by the dry method with 2
stages, namely alkalization and carboxymethylation.
Carboxymethyl formation is characterized by the
appearance of C = O peaks at wave number 1730
cm-1. Percent yield for hard and soft APE was 90%
and 83%, respectively. The surface morphology of
polysaccharide was change from smooth to wavy in
carboxymethyl. The degree of substitution of
carboxymethyl hard APE is smaller than that of soft
APE.
REFERENCES
Aspinall, G. O. 1959. Structural Chemistry of the
Hemicelluloses. In: WOLFROM, M. L. (ed.)
Advances in Carbohydrate Chemistry. Academic
Press.
Bento, J. F., Mazzaro, I., De Almeida Silva, L. M., De
Azevedo Moreira, R., Ferreira, M. L. C., Reicher, F. &
De Oliveira Petkowicz, C. L. 2013. Diverse patterns of
cell wall mannan/galactomannan occurrence in seeds
of the Leguminosae. Carbohydrate Polymers, 92, 192-
199.
Boual, Z., Pierre, G., Delattre, C., Benaoun, F., Petit, E.,
Gardarin, C., Michaud, P. & El Hadj, M. D. O. 2015.
Mediterranean semi-arid plant Astragalus armatus as a
source of bioactive galactomannan. Bioactive
Carbohydrates and Dietary Fibre, 5, 10-18.
Buriti, F. C. A., Dos Santos, K. M. O., Sombra, V. G.,
Maciel, J. S., Teixeira Sá, D. M. A., Salles, H. O.,
Oliveira, G., De Paula, R. C. M., Feitosa, J. P. A.,
Monteiro Moreira, A. C. O., Moreira, R. A. & Egito,
A. S. 2014. Characterisation of partially hydrolysed
Synthesis of Carboxymethyl Polysaccharide from Arenga Pinnata Polysaccharide and Monochloroasetic
67

galactomannan from Caesalpinia pulcherrima seeds as
a potential dietary fibre. Food Hydrocolloids, 35, 512-
521.
Cerqueira, M. A., Lima, Á. M., Teixeira, J. A., Moreira, R.
A. & Vicente, A. A. 2009. Suitability of novel
galactomannans as edible coatings for tropical fruits.
Journal of Food Engineering, 94, 372-378.
Gong, H., Liu, M., Chen, J., Han, F., Gao, C. & Zhang, B.
2012. Synthesis and characterization of carboxymethyl
guar gum and rheological properties of its solutions.
Carbohydrate Polymers, 88, 1015-1022.
Liyanage, S., Abidi, N., Auld, D. & Moussa, H. 2015.
Chemical and physical characterization of
galactomannan extracted from guar cultivars
(Cyamopsis tetragonolobus L.). Industrial Crops and
Products, 74, 388-396.
Moe, O. A. 1949. Carboxyalkyl ethers of carbohydrate
gums. Google Patents.
Mogea, J., Seibert, B. & Smits, W. 1991. Multipurpose
palms: the sugar palm (Arenga pinnata (Wurmb)
Merr.). Agroforestry Systems, 13, 111-129.
Niroomand, F., Khosravani, A. & Younesi, H. 2016.
Fabrication and properties of cellulose-nanochitosan
biocomposite film using ionic liquid. Cellulose, 23,
1311-1324.
Noleto, G. R., Petkowicz, C. L. O., Mercê, A. L. R.,
Noseda, M. D., Méndez-Sánchez, S. C., Reicher, F. &
Oliveira, M. B. M. 2009. Two galactomannan
preparations from seeds from Mimosa scabrella
(bracatinga): Complexation with oxovanadium(IV/V)
and cytotoxicity on HeLa cells. Journal of Inorganic
Biochemistry, 103, 749-757.
Orwa, C., Mutua, A., Kindt, R., Jamnadass, R. & Simons,
A. 2009. Agroforestree database: a tree species
reference and selection guide version 4.0. World
Agroforestry Centre ICRAF, Nairobi, KE.
Parvathy, K. S., Susheelamma, N. S., Tharanathan, R. N.
& Gaonkar, A. K. 2005. A simple non-aqueous
method for carboxymethylation of galactomannans.
Carbohydrate Polymers, 62, 137-141.
Singh, V., Sethi, R. & Tiwari, A. 2009. Structure
elucidation and properties of a non-ionic
galactomannan derived from the Cassia pleurocarpa
seeds. International Journal of Biological
Macromolecules, 44, 9-13.
Tarigan, J. B. 2014. Karakterisasi Edible Film Yang
Bersifat Antioksidan Dan Antimikroba Dari
Galaktomanan Biji Aren (Arenga pinnata) Yang
Diinkorporasi Dengan Minyak Atsiri Daun Kemangi
(Ocimum basilicum L.). Doktor Disertasi, Universitas
Sumatera Utara.
Tarigan, J. B., Barus, T., Kaban, J. & Marpongahtun.
Characteristic and Study of Antioxidant Activity
Galactomanan from "Kolang-Kaling" (Arenga
pinnata). Asian International Conference on
Materials, Mineral and Polymer, 23 - 24 March 2012
2012 Penang. Penang.
Tarigan, J. B., Kaban, J. & Zulmi, R. 2018a.
Microencapsulation of vitamin e from palm fatty acid
distillate with galactomannan and gum acacia using
spray drying method. IOP Conference Series:
Materials Science and Engineering, 309, 012095.
Tarigan, J. B. & Purba, D. 2015. Karakterisasi
Polisakarida Galaktomanan Kolang Kaling (Arenga
pinnata) Terikat Silang Fosfat. Majalah Polimer
Indonesia, 18, 1-8.
Tarigan, J. B., Purba, D. & Zuhra, C. F. 2018b.
Incorporation of vitamin E onto cross-linked
galactomannan phosphate matrix and in vitro study.
Asian Journal of Pharmaceutical and Clinical
Research, 11, 355-358.
Yang, L. & Zhang, L. M. 2009. Chemical structural and
chain conformational characterization of some
bioactive polysaccharides isolated from natural
sources. Carbohydrate Polymers, 76, 349-361.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
68
