
Furfural Synthesis from Mile-a-Minute Weed (Mikania micrantha)
using Roselle Petal Extract as Catalyst
Taslim
1*
, Iriany
1,2
, Okta Bani
1
, and Apri Wardiana Sinaga
1
1
Department of Chemical Engineering, Faculty of Engineering, Universitas Sumatera Utara, Jl. Almamater - Kampus USU,
Padang Bulan, Medan 20155, Indonesia
2
Center of Excellence for Natural Resources Based Technology, Universitas Sumatera Utara, Medan 20155, Indonesia
Keywords: Hydrolysis, mile-a-minute weed, roselle petal extract, furfural yield
Abstract: Mile-a-minute weed (Mikania micrantha) contains a large fraction of pentosan, and thus, is suitable as a
precursor of furfural. Generally, furfural production from biomass requires inorganic acids, such as sulfuric
acid and hydrochloric acid, as catalyst. However, the use of inorganic acid is not environmentally benign
and causes equipment corrosion. In this study, 50 g dry mile-a-minute weed was reduced to 100 meshes and
mixed with 50 g sodium chloride. Then, this mixture was added into roselle petal extract at a ratio of 1:6
(w/v) in a three-neck flask. The flask was then connected to a Liebig condenser and an erlenmeyer was
placed at the other end of the condenser to collect the furfural. The reaction was carried out at 100-120℃
and for 0-330 min. The resulting furfural was separated from water by extraction using chloroform. Two
layers of liquid were formed during extraction. The top layer was rich in water and the bottom layer was
rich in chloroform and furfural. The lower layer was distilled to purify furfural. The purified furfural was
identified by aniline acetate color test, Fourier-Tranform Infrared Spectrocopy (FTIR) and Gas
Chromatography–Mass Spectrometry (GCMS). The results suggested that organic acids from roselle petal
extraction can be used as a catalyst in furfural systhesis.
1 INTRODUCTION
The mile-a-minute weed has been known as one of
the most rampant invasive species in the world. This
plant has pentosane content reaching 56% and is
widespread throughout the Asia-Pacific region,
especially in Southeast China since 1980 (Ko et al.,
2013). It is listed as introduced, invasive, and
noxious plant according to United States Department
of Agriculture (USDA). For newly established
plantations such as those of tea, coffee, cocoa,
coconut, and palm oil, mile-a-minute is a threat to
the plant growth (Anderson et al., 2012). Control of
mile-a-minute is difficult and time-consuming, the
methods are also limited and expensive. It was
recommended cutting plants that grow on the ground
periodically to control their growth (Kuo, et al.,
2003). Thus, this plant needs to be utilized to reduce
the loss to many aspects of human life. One of its
utilization is as precursor of furfural (Taslim et al.,
2018).
Furfural is a furan derivative originating from
hemicellulose fraction of lignocellulose, which is
considered a promising biochemical based
commodity because its use allows production of
several products such as antacids, paints, fuel
additives, and fertilizers, as well as various other
products usually produced from non-renewable
resources (Guche et al., 2017).
Furfural is usually produced by hydrolysis with
aid of acid catalyst. Pentose dehydration is shown in
the following equation.
(C
5
H
8
O
4
)
n
+ nH
2
O → nC
5
H
10
O
5
(1)
pentosan pentose
nC
5
H
10
O
5
→ nC
5
H
4
O
2
+ 3nH
2
O (2)
pentose furfural
Furfural production and utilization will be useful
for reducing energy and environmental crises, as
well as increasing benefits of biorefinery economy
(Zhu et al., 2017).
Taslim, ., Iriany, ., Bani, O. and Sinaga, A.
Furfural Synthesis from Mile-a-Minute Weed (Mikania micrantha) using Roselle Petal Extract as Catalyst.
DOI: 10.5220/0008838400210025
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 21-25
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
21

Furfural synthesis from various biomass had
been reported in several literature using phosphoric
acid (Lenihan et al., 2010), sulfuric acid (Lacerda et
al., 2012), and hydrochloric acid (Hernandez-Salas
et al., 2009) as catalysts. The use of inorganic acids
can cause corrosion in the equipment used, and also
not environmentally benign. To overcome these
problems, organic catalysts is considered. The use of
organic acids derived from bilimbi as a catalyst in
furfural production had been carried out, in which
the yield reached 7.2% at 100℃ (Taslim et al.,
2018).
Until now, the use of organic acids from roselle
petals has never been reported. In fact, roselle flower
extract contains high organic acids including citric
acid, hydroxycitric acid, hibiscus acid, malic acid
and tartrate acid as the main compound, and oxalic
and ascorbic acid as minor compounds (Da-Costa-
Rocha et al., 2014). Therefore, this study aimed to
investigate the use of organic acids derived from
roselle petals as catalysts in furfural synthesis from
mile-a-minute weed, and compare it with sulfuric
acid catalyst.
2 MATERIAL AND METHOD
Mile-a-minute weed and roselle petals were obtained
from Medan Tuntungan, Indonesia. Chemicals used
such as sodium chloride, sulfuric acid, aniline, acetic
acid, and chloroform were purchased from Rudang
Jaya, Medan.
2.1 Sample Preparation
Sample preparation was carried out using a
procedure reported in the literature (Taslim et al.,
2018). A mix of mile-a-minute weed stems and
leaves were washed with water until free of dirt.
They were cut to ±1 cm using a knife. Then, they
were dried in the oven for 2 h at 100C. After
drying, mile-a-minute weed was milled in a ball
mill, and sieved to 100 meshes. Afterwards, the
sample was stored in a sealed plastic box at room
temperature and ready to be used for furfural
synthesis.
2.2 Extraction of Roselle Petals
Roselle extraction followed a procedure reported by
Taslim et al., (2008). Roselle petals were cleaned
using water to a constant pH, then grounded using a
commercial blender to get roselle extract. The
extract was filtered using Whatman paper number
41. Roselle filtrate was analyzed for its pH,
normality, acid number, and stored in a refrigerator
at 0C.
2.3 Synthesis of Furfural
Furfural synthesis followed a procedure reported
by Taslim et al., (2018) with modifications. As much
as 50 g mile-a-minute weed powder, 600 ml roselle
extract and 50 g sodium chloride were placed in a
three neck flask. The volume of the reaction mixture
was adjusted to 750 ml by adding distilled water.
The mixture was heated on a hot plate and stirred
using a magnetic stirrer. The flask was connected to
a long vertical pipe to provide sufficient length for
furfural steam passages. The long tube was
connected to a condenser to condense furfural vapor.
The reaction was carried out at 100, 110 and 120C
for 30, 60, 90, 120, 150, 180, 210, 240, 270, 300 and
330 min. The collected furfural-water mixture were
then extracted by adding 50 ml chloroform. After
extraction, two layers were formed, the top layer
was rich in water and the bottom layer was rich in
furfural and chloroform. The lower layer was
distilled at 60-70C to separate furfural from
chloroform. The purified furfural was collected and
its volume was recorded.
For comparison, the same procedure was carried
out using a 20% sulfuric acid catalyst. The furfural
obtained from these two types of catalyst were
identified by color assessment, FTIR, and GCMS.
3 RESULTS AND DISCUSSION
3.1. Preliminary Analysis
3.1.1. Preliminary Analysis of Mile-a-Minute
Weed
Preliminary analysis of mile-a-minute weed included
water content and pentosan content analysis. From
the results, mile-a-minute weed has an average
moisture of 80% and average pentosan content of
46%. These pentosan level is very close to the levels
reported in the literature (Ko et al., 2013; Taslim et
al., 2018).
3.1.2. Preliminary Analysis of Roselle Petal
Extract
Initial analysis of petal roselle catalyst extract
included pH, normality, and acid number. From the
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
22
results, the pH was 2.4, normality was 0.15 N, and
acid number was 6.93 g/g. For comparison, the 20%
sulfuric acid catalyst has pH of 0.2, normality of
2.27 N, and acid number of 222.83 g/g.
3.2. Effect of Reaction Temperature and
Reaction Time on Furfural Yield
The effect of reaction temperature and reaction
time on furfural yield is shown in Figure 1. In Figure
1, furfural formation catalyzed by roselle petal
extract was first observed at 30 min for reaction
temperature of 110 and 120
o
C. While for reaction
temperature of 100
o
C, furfural formation occurred at
reaction times above 90 min. However, after 300
min, the furfural yield at 100
o
C rivaled those of the
other temperatures. At 110 and 120
o
C, evaporation
rate of water was greater than furfural formation.
Pentosan in mile-a-minute weed was hydrolyzed to
pentose under acidic condition by the help of H
+
from roselle petal extract, then pentose dehydration
produced furfural as shown in equations (1) and (2).
Pentosan hydrolysis requires water while pentose
dehydration releases water. If water was evaporated
too quickly, hydrolysis will be incomplete and
pentose yield decreased. As a result, the furfural
yield may also decrease with increasing reaction
time and temperature. This causes furfural yield to
decrease at 330 min reaction time and reaction
temperatures of 110 and 120
o
C. Thus temperature
control is very necessary in this reaction to ensure
that these two stages of reaction take place well.
From figure 1, furfural yield produced using
roselle petal extract catalyst is still lower than that
using sulfuric acid catalyst. This is because the acid
number of sulfuric acid is 32 times greater than the
acid number of roselle petal extract, which means
that H
+
ions in sulfuric acid are more concentrated
than H
+
ions in roselle petal extract. The highest
furfural yield of 4.8% was obtained at reaction time
of 330 min, and reaction temperature of 100
o
C. The
optimum temperature reported by previous
researchers was 100
o
C, both for furfural formation
from pentose sugar using formic acid (Kim et al.,
2016) and for furfural formation from mile-a-minute
weed using organic acid catalysts from bilimbi
Taslim et al ., 2018).
At constant temperatures, increasing reaction
time increases furfural yield due to longer contact
times. The use of roselle petal extract in furfural
production does not require high temperatures, but
requires a long time of around 300 min.
In this study, furfural yield obtained using a 20%
sulfuric acid catalyst was up to 6.4%. This yield is
lower than the yield reported by other researcher at
8.3% under similar condition, but using corn cobs as
precursor (Shaffeeq et al., 2015).
0
1
2
3
4
5
6
7
0 60 120 180 240 300 360
Yield (%)
Time (minute)
Roselle, 100 C
Roselle, 110 C
Roselle, 120 C
H SO , 120 C
Figure 1: Effect of reaction time on furfural yield at
various temperatures
3.3. Furfural Analysis
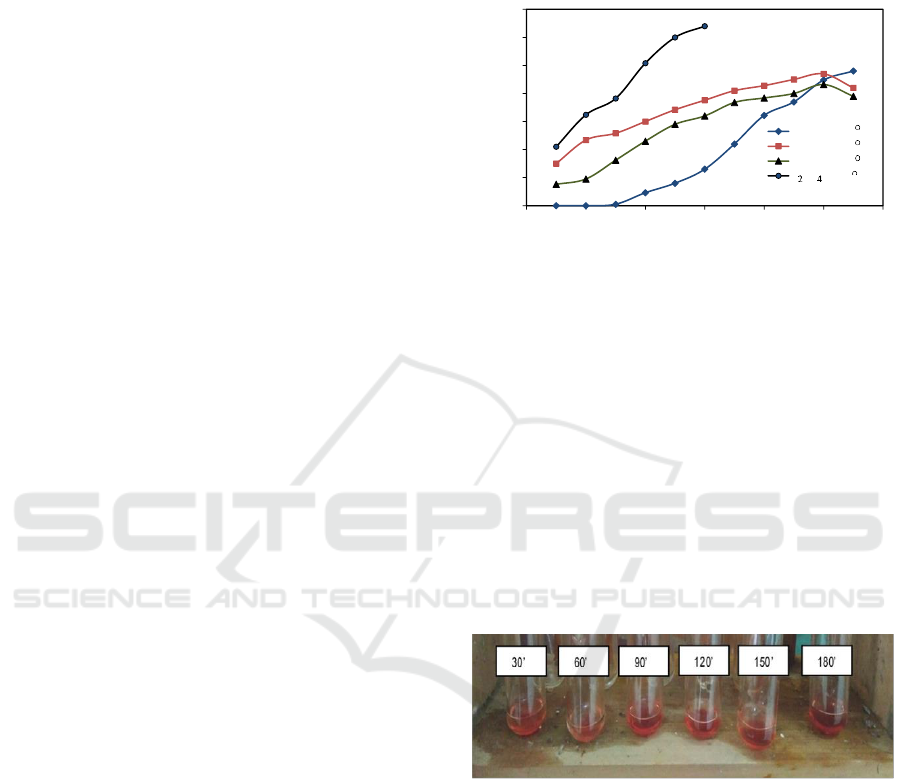
3.3.1. Identification of Furfural by Colour
Assessment
In this study, the qualitative identification of furfural
was done using aniline acetate reagent at aniline and
acetate ratio of 1:1 (v/v). The change in sample color
from clear to red indicates the presence of furfural.
In figure 2, the red color is still pale, indicating that
furfural formed using a roselle petal extract catalyst
was not much.
Figure 2: Colour assessment of furfural produced using
roselle petal extract catalyst at 120
o
C, at different reaction
time
In figure 3, the darker red color indicates that
more furfural was formed using sulfuric acid
catalysts. Sample at 180 min (3 h) appears darker,
indicating more formation of furfural. As shown in
figure 3, all samples became dark red after reaction
time of 30 min. This suggests that for sulfuric acid
catalyst, a long reaction time is not required. Similar
result was also reported in the literature (Taslim et
al., 2018).
Furfural Synthesis from Mile-a-Minute Weed (Mikania micrantha) using Roselle Petal Extract as Catalyst
23
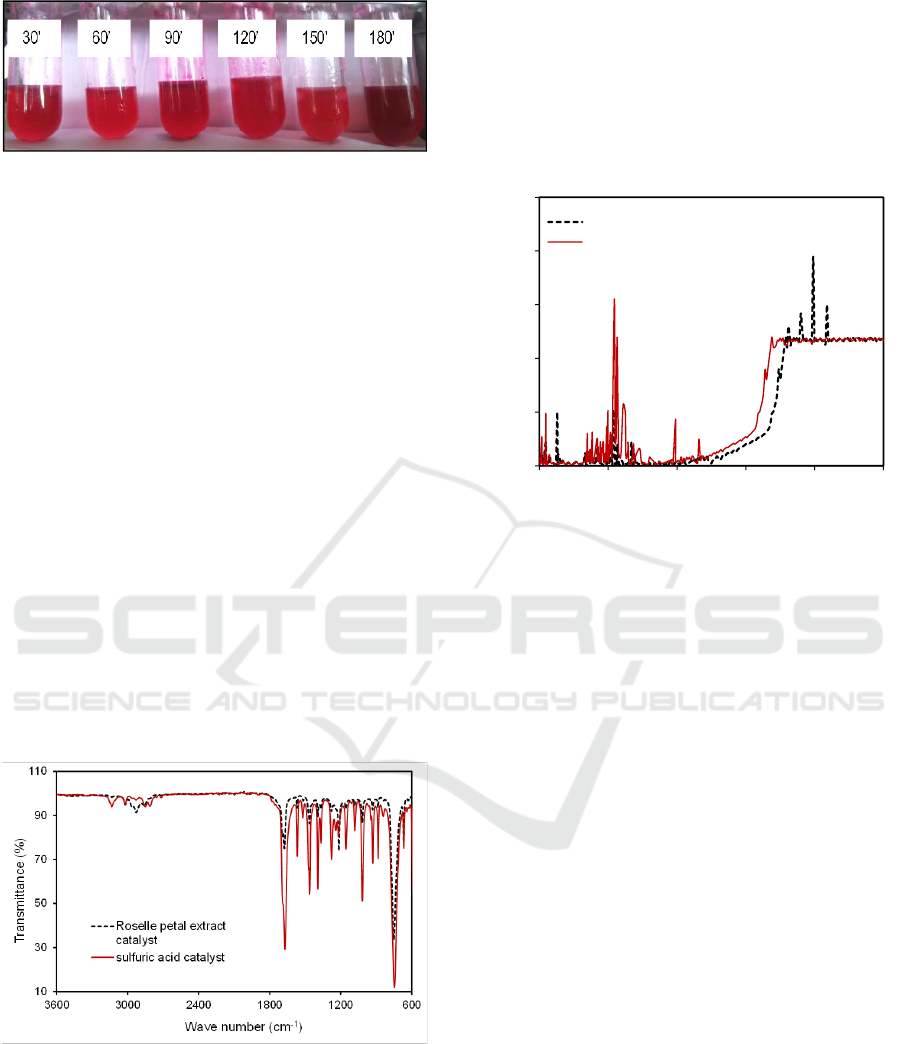
Figure 3: Colour assessment of furfural produced using
sulfuric acid catalyst at 120
o
C, at different reaction time.
3.3.2. FTIR Analysis
The result of furfural analysis using FTIR is
shown in figure 4. Based on IR spectra (Figure 4)
aldehyde group in furfural was observed as
evidenced by C=O stretching vibration (1700-1600
cm
-1
) and CH aldehydes (2860-2800 cm
-1
) refleted
by the peak at 1674.52 cm
-1
and 2851.07 cm
-1
respectively for sulfuric acid catalyst and roselle
petal extract catalyst. The apearance of C=C
aromatic stretching vibration (1600-1475 cm
-1
) was
evidenced in the area around 1521.45 cm
-1
.
Stretching vibration peak at around 1166.00 on
sample using roselle petal extract catalyst indicated
C-O-C bonds (1200-1100 cm
-1
) within the molecular
structure of furfural, while for sulfuric acid catalyst,
same occurrence was observed at vibration peak of
1176 cm
-1
. The spectra of the compound produced
from mile-a-minute weed hydrolysis was virtually
identical to standard vibrations of furfural (Ong and
Sashikala, 2007). Therefore, it can be stated that the
compound was furfural.
Figure 4: FTIR spectra of furfural produced using roselle
petal extract and sulfuric acid catalyst
3.3.3. GCMS Analysis
Figure 5 shows the results of analysis using GCMS.
These results confirm that furfural has been formed
as a result of mile-a-minute weed ahydrolysis. For
the roselle petal extract catalyst, furfural compound
was identified at peak 2 (retention time of 3.37 min)
as 2,5 furandione, 3-ethyl-4-methyl. For sulfuric
acid catalyst, furfural compound was identified at
peak 3 (retention time of 3.28 min) as 2,5
furandione. Furandione belongs to the furfural
group, which proves that the samples contained
furfural.
27
22
1
2
3
14
12
30
11
31
34
20
4
13
6
5
9
7
41
20
44
23
21
24
47
8
35
46
40
10
0
5
10
15
20
25
3 8 13 18 23 28
Intensity x 10
4
minute
Roselle petal extract catalyst
Sulfuric acid catalyst
Figure 5: GCMS analysis of furfural produced using
roselle petal extract and sulfuric catalyst
4 CONCLUSIONS
Roselle petal extract can be used as a catalyst in
furfural synthesis from mile-a-minute weed. The
highest furfural yield of 4.8% using the roselle petal
extract was obtained at reaction temperature of
100
o
C and, reaction time of 330 min. This yield is
still lower than that using a sulfuric acid catalyst
which reached 6.4% in 150 min. Colour assessment,
FTIR, and GCMS analysis indicated the presence of
furfural as a result of mile-a-minute weed
hydrolysis.
REFERENCES
Anderson, P.J., Weaver, R.E., Neubig, K.M., Frank, M.S.,
Dixon, W.N., 2012. Which mikania: Native nine or
noxious weed? Botany Circular 37, 1-6.
Da-Costa-Rocha I., Bonnlaender B., Sievers H., Pischel I.,
Heinrich M., 2014. Hibiscus sabdariffa L. – A phyto-
chemical and pharmacological review. Food
Chemistry 165, 424-443.
Guche, J.E.O., Ameh, A.O, Tanimu, Y., Egu. S.A., 2017.
Determination and optimization of effect of process
parameters on furfural yield from microalgae. FUW
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
24

Trends in Science and Technology Journal 2(2), 782-
787.
Hernandez-Salas, J.M., Villa-Ramirez, M.S., Veloz-
Rendon, J.S., Rivera-Hernandez, K.N., Gonzalez-
Cesar, R.A., Plascencia-Espinosa, M.A., Trejo-
Estrada, S.R., 2009. Comparative hydrolysis and
fermentation of sugarcane and agave bagasse.
Bioresource Technology 100, 1238–1245.
Ko, C.H., Shih, T.L., Jhan, B.T., Chang, F.C., Wang,
Y.N., Wang, Y.C., 2013. Production of xylo-
oligosaccharides from forest waste by membrane
separation and Paenibacillus xylanase hydrolysis.
BioResources 8, 612-627.
Kuo, Y.L., 2003. Ecological characteristics of three
Invasive Plants (Leucaena leucocephala, Mikania
micrantha, and Stachytarpheta) in Southern Taiwan.
In: Oka, M., Matsui, M., Shiomi, T., Ogawa, Y.,
Tsauchiya, K (Eds), Biological invasions-
environmental impacts and the developments of a
database for the Asia-Pacific region. Proceeding of the
NIAES-FFTC Joint International Seminar, Tsukuba,
Japan, 177-191.
Kim, T. H., Ryu H.J., Oh K.K., 2016. Low acid
hydrothermal fractionation of giant miscanthus for
production of xylose-rich hydrolysate and furfural.
Bioresource Technology 218, 367–72.
Lacerda, T.M., de Paula, M.P., Zambon, M.D., Frollini, E.
Saccharification of Brazilian sisal pulp: evaluating the
impact of mercerization on non-hydrolyzed pulp and
hydrolysis products. Cellulose 19, (2012): 351–362.
Lenihan, P., Orozco, A., O’Neill, E., Ahmad, M.N.M.,
Rooney, D.W., Walker, G.M., 2010. Dilute acid
hydrolysis of lignocellulosic biomass. Chemical
Engineering Journal 156, 395–403.
Ong, H K, Sashikala M., 2007. Identification of furfural
synthesized from pentosan in rice husk. Journal of
Tropical Agriculture and Food Science 35 (2): 305–
12.
Shafeeq, A., Muhammad, A., Sarfaraz S., Akram Z.,
H.M.U. Saeed H.M.U., Farooq U., 2015. Effect of
acid concentration on the extraction of furfural from
corn cobs. International Journal of Chemical
Engineering and Application 6, 381-386.
Taslim, Mirnandaulia M., Iriany, Tambun R., 2018.
Furfural production from mile-a-minute weed
(Mikania micrantha) using organic acid from bilimbi
(Averrhoa bilimbi). Asian Journal of Chemistry 30(5):
1007-1011.
Zhu, Y., Li, W., Lu Y., Zhang T., Jameel H, Chang H.M.,
Ma, L., 2017. Production of furfural from xylose and
corn stover catalyzed by a novel porous carbon solid
acid in -valero lactone. Royal Society of Chemistry
Advances 7: 29916-29924.
Furfural Synthesis from Mile-a-Minute Weed (Mikania micrantha) using Roselle Petal Extract as Catalyst
25
