
Mangan Coated N-Graphene for Good Performance Electrode in
Primary Battery Anode
Rikson Siburian
1*
, Sabarmin Perangin-angin
1
, Helmina Sembiring
1
, Crystina Simanjuntak
2
and Yopi Sihombing
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
2
Postgraduate Chemistry Study Program, Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara,
Jl. Bioteknologi No. 1 Kampus USU, Medan, Indonesia
yopivani17@gmail.com
Keywords: N-Graphene, Mn/N-Graphene, Alternative Electrode, Primary Battery.
Abstract: Even though lithium is expected to remain the dominant battery technology for the next decade, new battery
technologies are on their way. N-Graphene and Mn/N-Graphene have the potential to be used as an alternative
electrode on primary battery anode. The results showed that the N-Graphene diffraction showed a weak and
wide peak at 2θ = 26.5
o
which indicates that N-Graphene was formed. The data is consistent with EDX data,
where the abundance of N atoms in N-Graphene (4.68%). At the Mn / N-Graphene electrode a sharp peak
appears at 2θ = 31
o
, indicating that Mn is deposited in N-Graphene. The EDX data also shows that Mn atoms
are deposited in graphene (0.15%). Mn / N-Graphene has the highest conductivity value (1250 μS/ cm)
compared to primary battery anodes (10 μS / cm), Graphite/N-Graphene (350 μS / cm) and N-Graphene
(1157.33 μS / cm).
1 INTRODUCTION
Graphene, the type of carbon in the form of
monolayer graphite, has a specific surface area of
2,600 m
2
/g (Stoller et al., 2008) with a honeycomb-
shaped structure that has the potential to produce
higher lithium ion storage capacity (Eriksson, 2001),
high electron mobility of 15,000 cm
2
/Vs (Geng et al,
2011), extraordinary thermal conductivity of 3000
W/mK (Netro et al, 2009) and has good chemical
stability and mechanical properties (Bolotin et al.,
2008). The advantages of graphene is an interesting
thing to form a composite material that is used as an
electrode material on lithium ion batteries. However,
pure graphene has poor pore affinity, low coulomb
efficiency, high charge-discharge platform and low
cycle stability so that it cannot be a direct substitute
for carbon-based commercial electrode material in
lithium ion batteries (Atabaki and Kovacevic, 2013).
To overcome the poor pore affinity of graphene a
doping is needed to fix it (Yang et al, 2015). A doping
agent suitable for improving the pore affinity of
graphene is nitrogen (Yu et al, 2013; Sun et al, 2012).
Nitrogen will modify the graphene structure so that it
will strengthen the stability of graphene in each bond
and improve porosity (Xing et al, 2016). Depositing
Pt metal to graphene will improve the electrical
properties and catalytic activity of Graphene (Rikson,
2014). Therefore, research is needed to improve the
quality of primary batteries by combining Mn metal
with N-graphene. The Mn metal used has an electrical
conductivity of 6.2. 105 (S / m).
2 MATERIALS AND METHODS
2.1 Synthesis Graphite/N-Graphene
About 1 g of graphite was added to the glass beaker
is then added 200 mL Ethanol Absolute, stirred for 1
hour. Then, 1 g of N/graphene was added with 200
mL of absolute Ethanol, stirred for 1 hour. The
graphite-ethanol mixture was added into N/graphene-
ethanol, and then stirred for 2 hours. Filtered using
Whatmann no.42, then the precipitate was dried with
an oven at 80
o
C and characterized by XRD.
18
Siburian, R., Perangin-angin, S., Sembiring, H., Simanjuntak, C. and Sihombing, Y.
Mangan Coated N-Graphene for Good Performance Electrode in Primary Battery Anode.
DOI: 10.5220/0008838300180020
In Proceedings of the 1st International Conference on Chemical Science and Technology Innovation (ICOCSTI 2019), pages 18-20
ISBN: 978-989-758-415-2
Copyright
c
2020 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
2.2 Synthesis Mn/N-Graphene
About 1 g of MnCl
2
was added to the glass beaker is
then added 200 mL Ethanol Absolute, stirred for 1
hour. Then, 1 g of N/graphene was added with 200
mL of absolute Ethanol, and then stirred for 1 hour.
The MnCl
2
-ethanol mixture was added into
N/graphene-ethanol then stirred for 2 hours. Filtered
using Whatmann no.42, then the precipitate was dried
with an oven at 80
0
C and characterized by XRD.
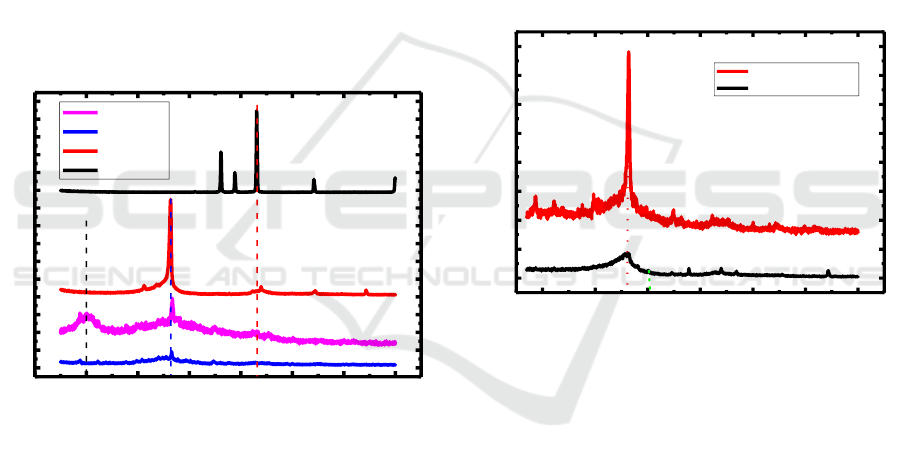
3 RESULTS AND DISCUSSIONS
3.1 Diffractogram of Zinc Anode,
Graphite, Graphene and
N-Graphene
X-ray diffraction data from primary battery anodes,
graphite, graphene and N-graphene are shown in
Figure 1.
Figure 1: Diffractogram of Zinc anode, graphite, graphene
and N-graphene.
The data above shows the XRD diffraction pattern
of commercial primary battery anodes where there are
sharp peaks and densities which are at 2θ = 43
o
Zn
(101), graphite and graphene show peaks at 2θ = 26.5
o
which correspond to diffraction lines C (002) , where
the sharp and tight peaks on the graphite diffraction
pattern indicate that the particle size of graphite is
large and overlapping and graphene has a weak and
wide peak which indicates the particle size of nano-
sized graphite and stacked on the interlayer of
graphene. The occurrence of peak changes from the
diffractogram is due to the bonding of graphite and
oxidation and the entry of oxygen into the interlayer
space in graphite (Jeong et al, 2008).
X-ray diffraction pattern of N-graphene which
shows the peak at 2θ = 10
o
there is a fairly sharp but
wide peak indicating that N from ammonia (NH
3
) has
been deposited into graphene. The above diffraction
pattern also shows a peak that is at 2θ = 26.5
o
which
corresponds to the diffraction line C (002), which has
a tightly sharp peak and is surrounded by widened
peaks which indicate that the particle size is nano-
sized and stacked graphene on the interlayer of
graphene which has been successfully synthesized.
This data explains that N-graphene has been produced
which is a modification of a graphene by doping N
from ammonia into graphene.
3.2 Diffractogram of Graphite/
N-Graphene and Mn/N-Graphene
X-ray diffraction data from primary graphite/N-
graphene and Mn/N-graphene are shown in Figure 2.
Figure 2: Diffractogram of Graphite/N-Graphene and
Mn/N-Graphene.
The XRD diffraction pattern of graphite / N-graphene
shows sharp and tight peaks at 2θ = 26,5
o
C (002)
which are specific to the intensity of carbon atoms
which are still dominated by hexagonal graphite
phases. Diffractogram changes indicate that N-
graphene has been deposited into graphite. The XRD
diffraction of Mn / N-Graphene shows a broad and
weak peak at 2θ = 26.5
o
C (002) which is specific to
the intensity of carbon atoms which is still dominated
by the hexagonal phase and diffraction at 2θ = 31
o
Mn
(100) which indicates that Mn is deposited into N-
graphene (Jeong et al, 2008).
4 CONCLUSIONS
The electrode synthesis in the primary battery anode
with graphite becomes graphene into N-graphene
0 10 20 30 40 50 60 70
Intensity (a.u)
2 Theta
N-Graphene
Graphene
Graphite
Zinc Anode
43
o
Zn (101)
10
o
N
26,5
o
C (002)
10 20 30 40 50 60 70
Intensity (a.u)
2 Theta
Grafit/N-Grafena
Mn/N-Grafena
31
o
Mn (100)
26
o
C (002)
Mangan Coated N-Graphene for Good Performance Electrode in Primary Battery Anode
19

using a modified Hummer method followed by
depositing Manganese metal on the N-graphene
compound. Where N-graphene is produced by
deactivating graphene using ammonia (NH
3
) so that
graphene is reduced. The deformed graphene is
modified and forms N-graphene which has a good
catalyst activeness value and has a high conductivity
value.
ACKNOWLEDGEMENTS
Authors would like to thankful to Ministry of
Research, Technology and Higher Education,
Republic of Indonesia who supported funding of our
research by the research grant: DRPM research,
Universitas Sumatera Utara No.
157/UN5.2.3.1/PPM/KP-DRPM/2019.
REFERENCES
Atabaki dan Kovacevic, 2013, The electronic Properties of
Graphene, Rev. modern Physic Journal of the American
Physical Society.
Bolotin, K.I, Sikes, K.J, Jiang, Z., Klima, M., Fudenberg,
G., Hone, J., Kim, P. dan Stormer, H.I., 2008, Ultrahigh
Electron Mobility in Suspended Graphene, Solid State
Commun, 146 (1) : 351-355.
Eriksson, T., 2001, LiMn2O4 as a Li-Ion Battery Chatoda
from Bulk to Electrolyte Interfase. Uppsala University,
Sweden
Geng., D., Songlan Y., Yong Z., Jinli Y., Jian L., Ruying
L., Tsun K. S., Xuenliang S., Siyu Y. and Shanna K.,
2011, Nitrogen Doping Effects on the Structure of
Graphene, Applied Surface Science 257 (2011) : 9193–
9198.
Jeong, K-Hae. Lee, P.Y. Lahaye, R. J. W. E. Park, H-Min.
An, K. H. Kim, I. J. Yang, Woong-Cheo. Park, C.Y.
Ruoff, R. S. Lee, Y. H. 2008. Evidence of Graphitic AB
Stacking Order of graphit oxides. J. AM. Chem. SOC.
American chemical society.
Netro, C.A.H., Guinea, F,. Peres, N.M.R., Novoselov, K.S.
dan Geim, A.K., 2009, The Electronic Properties of
Graphene Rev. Modern Physics, Journal of The
American Physical Society, 81 (1): 109-162.
Siburian, RA.F. 2014. Support Material Effect For Pt
Catalytic Activity at Cathode. University of Nusa
Cendana. Kupang
Stoller, M.D., Park, S.J., Zhu, Y.W., An, J.H. dan Ruoff,
R.S., 2008, Graphene-Based Ultracapacitors, Nano
Lett, 8 (1): 3498-3502.
Sun, L., Lei W., Chungui T. Taixing T., Ying X., Keying
S., Meitong L. and Honggang F., 2012, Nitrogen-
Doped Graphene With High Nitrogen Level Via A One-
Step Hydrothermal Reaction of Graphene Oxide with
Urea for Superior Capacitive Energy Storage, RSC
Advances, 2 (1) : 4498–4506.
Xing, Z., Zhicheng J., Yulong Z., Jialu W., Yabo Z.,
Yinghuai Q. and Yitai Q., 2016, One-pot hydrothermal
synthesis of Nitrogen-doped graphene as
highperformance anode materials for lithium ion
batteries, Scientific Reports, 6 (26146):1-10.
Yang, S., Yuan L., Xuefeng S., Peng Z. and Lian G., 2015,
Covalently Coupled Ultrafine H-TiO2
Nanocrystals/Nitrogen-Doped Graphene Hybrid
Materials for High-Performance Supercapacitor, ACS
Applied Materials & Interfaces, 1 (1) :1-26
Yu. F., Lo S. T., Lin J. C., Zhang W., Lu J. Y., Liu F. H.
and Li L. J., 2013, Nitrogen Doped Graphene Sheets
Grown by Chemical Vapor Deposition: Synthesis and
influence of nitrogen impurities on carrier transport,
ACS nano, 7(8) : 6522-6532.
ICOCSTI 2019 - International Conference on Chemical Science and Technology Innovation
20
