
Dietary Intakes Influence on Metallomic Distribution in Vital Organs
and Their Implications
Solomon W. Leung
1
, Brad Williams
2
and James C. Lai
3
1
Civil and Environmental Engineering Department and Measurement and Control Research Center, Idaho State University,
Pocatello, Idaho, 83209, USA
2
Civil and Environmental Engineering Department, Idaho State University, Pocatello, Idaho, 83209, USA
3
Biomedical and Pharmaceutical Sciences Department, Idaho State University, Pocatello, ID 82309 USA
Keywords: Elements Distribution, Mn, Nutrition Intake, Vital Organs
Abstract: The intake and concentration of metals and electrolytes in our diet are believed to be affecting our general
health, in particular, the proper functions of vital organs. For example, in addition to other genetic and
environmental factors, consuming water with high alkalinity for a prolonged time is suspected of leading to
diseases such as kidney stones. There is evidence that elemental accumulation due to excessive metal
intakes would lead to organ failure. This study is an extensive investigation of metallomic distribution in
Wistar Rats after they have consumed 30 different elements (including heavy metals and electrolytes) via
dietary intakes throughout their lifespan (from 5 to 750 days). In this study, the distributions of these
elements in various vital organs such as heart, kidney, lung, spleen, liver, pituitary, and uterus over time
were analyzed. In addition, how heavy metal supplement, such as Mn, influenced the elemental
accumulations inside the organs was also conducted. This study has high impact to our understanding of
how the environment would affect our well beings. This study would provide insights on how our diet
would affect the accumulations of unwanted elements, such as heavy metals, in our vital organs. The results
may also help researchers and health practitioner to identify possible links between daily diet and associated
diseases inside the vital organs.
1 INTRODUCTION
Since the era of industrial evolution, living
conditions for human have improved drastically;
with the consequence of better living conditions and
less physical activities, we are facing other aspects
of health issues such as obesity and hyper immunity
responses (allergy). In modern living, considerable
attention has been paid to dietary intake or
supplement due to health concerns. On the other
hand, involuntarily consumption of unwanted
chemicals and preservatives via processed foods and
polluted water is also possible. One such example
would be consumption of drinking water source that
is laden with soluble ions and heavy metals. This
occurs quite frequently for those that are living in
rural areas with no proper treatment system for their
drinking water in developing countries.
Attempts in understanding the homeostasis of
different elements in brain and other major organs
fell short significantly due to the vast complexity of
the mechanisms involved (Pardridge, 2003). What
cause this complexity are the multiple factors that
can affect the dynamics of biological functions. For
examples, an element of different compounds
(chloride versus phosphate) would have various
uptake rates (Anderson et al., 2008) and the uptake
rate of elements in solution by the digestive system
was proven to be faster than in food stuffs.
Elemental accumulations are not solely related to
exposure but likely have more to do with
impairment of the relevant homeostasis mechanism
(Bolognin et al., 2009); conversely, a known Cu
deficient sample of mice were able to be revised by
supplementation of Cu in drinking water (Bayer et
al., 2003). In addition, larger sample size is needed
to observe statistical significance in the small
changes over lifetime exposure (Maynard et al.,
2009). Such constraint creates a vast financial and
operational obstacle to conduct research, and the
hurdle is more difficult if human subject is involved.
Currently, the understanding of homeostasis
mechanisms for accumulation and control of
individual element in our body is very limited,
30
W. Leung, S., Williams, B. and C. Lai, J.
Dietary Intakes Influence on Metallomic Distribution in Vital Organs and Their Implications.
DOI: 10.5220/0008654100300036
In Proceedings of the International Conference on Future Environment Pollution and Prevention (ICFEPP 2019), pages 30-36
ISBN: 978-989-758-394-0
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
hence, the influence of similar elements to each
other is practically non-existent. However, the result
of Zn replacing Cu in a competitive homeostasis
mechanism in rat’s brain was recently reported by
Maynard et al. (2009). Therefore, it can be
concluded that our understanding of elemental
homeostasis mechanisms in our body systems is still
in the beginning stage, and the opportunities for
more research and development are immensely
available.
There are chemicals (vitamins, for example) and
elements and trace elements that we need to
maintain proper bodily functions; in this study, we
focused on the essence of major and selected trace
elements and how these elements accumulated in our
major organs. The major organs are: heart, kidney,
lung, spleen, liver, pituitary, and uterus. Information
matrices of these 30 different elements (including
heavy metals and some electrolytes) that were fed to
rats as part of a regular diet were obtained from the
seven major organs and analyzed. Furthermore, we
fed the rats with various concentrations of Mn (as a
surrogate of heavy metal) and observed how these
30 elemental accumulations in the vital organs were
affected by the different consumptions of Mn in the
diet as an adult (120 days).
2 EXPERIMENTS AND
PROCEDURES
The 30 elements included in this study were: Al, As,
Ba, Br, Ca, Cd, Cl, Co, Cr, Cu, F, Fe, Hg, I, K, La,
Mg, Mn, Mo, Na, Rb, S, Sb, Sc, Se, Si, Sm, Sr, V,
and Zn. Analytical measurements of the elements
from the organs of adult (120 days) Wistar rats and
diet pellets that were used to feed the rats were
reported in a previous paper (Wright et al.). Table 1
lists the elements of the rat food pellet detectable by
Instrumental Neutron Activation Analysis (INAA),
elements that were from the 30 interested elements
but not listed in Table 1 were not detectable by
INAA due to low concentration. All values listed
are means ± standard deviations of the 20 detectable
elements. ND indicates not detectable, and values in
brackets are the maximum elemental concentrations
present in the pellet diet.
The main analysis for this paper is to compare
elemental accumulations in the said organs above in
relations to control adult rats and Mn-treated adult
rats. The Mn dosages of control and three different
adult groups are listed as follows:
1. Control samples taken from 120- days-old
adult female Wistar Rat.
2. Group A adult with life-long manganese
treatment with 1 mg/ml MnCl
2
·4H
2
O in drinking
water.
3. Group B adult with life-long manganese
treatment with 10 mg/ml MnCl
2
·4H
2
O in drinking
water.
4. Group C adult with life-long manganese
treatment with 20 mg/ml MnCl
2
·4H
2
O in drinking
water.
Table 1: Elemental concentration of rat food pellet
Element
Concentration
Ca
mg /g
6.393
1.68
Cl
mg /g
2.305
0.485
Fe
mg /g
0.25
0.051
K
mg /g
5.362
0.951
Mg
mg /g
1.168
0.322
Na
mg /g
1.475
0.32
Al
gg
98.06
8.05
Br
gg
10.57
2.26
Co
gg
0.2
0.04
Cr
gg
1.5
0.44
Cu
gg
7.21
2.12
Fe
gg
ND(5.0)
Hg
gg
ND(.25)
I
gg
ND(.5)
Mn
gg
49.34
8.65
Mo
gg
2.8
0.45
Se
gg
ND(.25)
Rb
gg
12.73
2.61
V
gg
ND(.5)
Zn
gg
47.73
4.11
3 RESULTS
3.1 Heart
Mercury (Hg) is most drastically impacted by Mn
treatment. Hg content has increased about 18 times
from the control concentration. Along with the
increase in Hg, there is higher Hg concentration with
higher dosages of Mn treatment. Hg concentration
Dietary Intakes Influence on Metallomic Distribution in Vital Organs and Their Implications
31
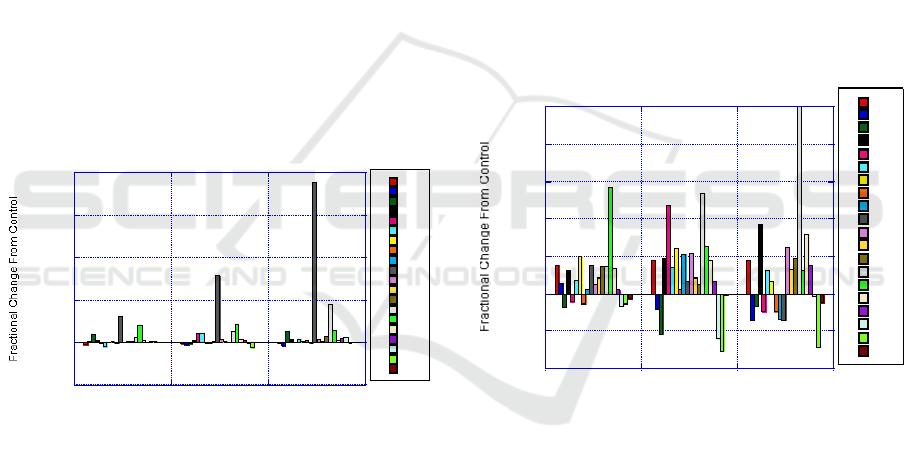
in the heart is showing an impact with prolonged Mn
intake. Molybdenum (Mo) also had a large increase
in concentration from control levels. An interesting
effect is with Calcium (Ca). From the A dosage
level Ca increased almost 100% but then on the B
dosage level Ca slightly decreased. Then Ca
increased again on the C dosage level.
The impact of Mn on Hg is an important point to
highlight. Mn has been known to be present in
drinking water and also used to treat drinking water
that would lead to Mn ingestion. There are known
harmful elements that will greatly increase in
accumulation in the body by Mn ingestion.
Increased Hg in the heart is a concern also because
Hg is a well-known neurotoxin. Its impact on the
heart directly may not be clear but there are other
known adverse effects on the brain, nervous system,
and kidneys.
In general, above normal elemental accumulation
in the heart can lead to functional loss or heart
failure. The risk of heart failure by increased
accumulation of heavy metal in itself is worth
additional study and investigation. From this
analysis, increases in heavy metal’s presence that are
induced by Hg may contribute to heart failure.
-5
0
5
10
15
20
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 1: Results of heart with Mn treatment for Group A,
B, and C as compared with control.
3.2 Kidney
For Mn treatment, the kidney showed increases in
Mn but also showed decreases in V. Mo increased
for treatment dosage A but then decreased to
treatment dosage C. Cobalt (Co) showed an increase
on treatment dosage B but very little change for A
and C. Chlorine (Cl) increased with increased Mn.
Cl reached nearly a 100% increase for treatment
dosage C. Mn impacts electrolyte’s balance.
The observations worthy of discussion are that
Mo showed an increase of over 100% by introducing
Mn at the dosage level A. But then as dosage levels
increase the amount of change for Mo decreases.
This suggests a competition mechanism between Mn
and Mo for retention or accumulation in the kidney.
Very few elements showed decrease in
accumulation due to Mn treatment. This would
suggest that Mn increases accumulation of the
majority of the elements in the kidney. Also similar
to Mo, the lower dosage of group A had the least
amount of decrease in accumulation from control for
all the elements. Then with increased Mn intake, the
elemental accumulation either increases or decreased
more than those obtained with the lower Mn dosage.
This suggests that Mn has a concentration-related
impact on accumulation.
The kidney controls electrolyte balance to collect
or accumulate excessive levels of different elements
that may be present in the body. By introducing Mn
treatment, this also increases the overall elemental
accumulation compared to other organs. Next step
analysis would be a mass balance and how well the
kidney flushes elevated concentrations from the
body.
-1
-0.5
0
0.5
1
1.5
2
2.5
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 2: Results of kidney with Mn treatment for Group
A, B, and C as compared with control.
3.3 Lung
For the lung, Hg also shows significant increase
from control. Treatment dosage B showed the
highest Hg levels but dosage C induced almost a
300% increase. Many elements showed at or above
50% increase for the highest Mn dosage with all of
them becoming increasingly concentrated with
increased Mn dosage. Mn treatment shows evidence
that there is a wide range of elements that have
concentration impacts related to Mn.
Mn ingested by diet has a very large impact on
elemental accumulation of the lungs. This
relationship is extremely important because one of
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
32
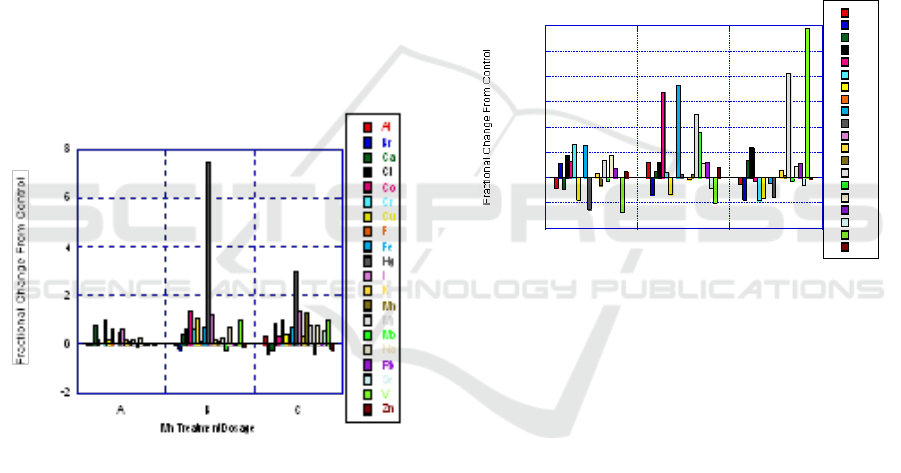
the most common methods of exposure and intake is
by the lungs and breathing air. From this data set, a
link to practical use is that an increased Mn intake
may allow the subject to be at higher risk for toxic
elemental accumulation in the lungs that could lead
to illness and other health concerns.
This data shows effect via ingestion then through
the blood to the lungs which is different than most
studies that look at inhalation and the accumulation
by inhalation. What is shown by this analysis is that
orally ingested Mn can increase the accumulation of
elements ingested orally. For further investigation,
the relationship between orally ingested Mn and the
accumulation of elemental exposure by inhalation
would be important. Miners and similar type of
industrial workers are often exposed to highly
elevated inhalation of different elements. This link
between orally ingested Mn and elemental exposure
by inhalation could greatly impact mining personnel
protective equipment and medical treatment of
miners. Current exposure limits are debatable and
may need to be adjusted with consideration of
possible Mn ingestion.
Figure 3: Results of lung with Mn treatment for Group A,
B, and C as compared with control.
3.4 Spleen
Mn shows increased concentration compared to
control for increased Mn dosages indicating a
possible correlation. V has drastic variations going
from nearly 75% less than control to nearly 3 times
the control value. Co shows an increased
concentration for only one dosage but almost no
change for the other dosage treatments. Iron (Fe)
shows elevated concentration for dosage A and then
decreases to negative values for the largest Mn
dosage.
The fractional change from control is very
sporadic with the spleen. At one dosage level a
particular element will show an increase and then at
a different dosage show a decrease. Cr is one
element that at the small Mn dosage shows a large
increase but with increased Mn the fractional change
of Cr goes negative. At a low dosage more Cr is
accumulated and at a high dosage less Cr is
accumulated. This suggests that starting with low
Mn treatment creates a mechanism allowing more Cr
to accumulate. Then as Mn increases it competes
with and displaces Cr to levels even below the
control.
In the case of Cu, treatment with Mn reduces
accumulation. The reduced accumulation is
relatively the same at all three Mn concentrations.
This suggests that Cu has an interaction with Mn but
is not correlated to Mn dosage concentration.
-1
-0.5
0
0.5
1
1.5
2
2.5
3
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 4: Results of spleen with Mn treatment for Group
A, B, and C as compared with control.
3.5 Liver
The liver is not showing any individual elements
that have drastic increases as compared to organs
discussed in previous sections. Of all the elements
there are only five that show greater than a 50%
change and only one element that shows over a
100% change. Cr shows an increasing trend that
relates to increased Mn dosage. Decreases in
Rubidium (Rb) and Iodine (I) were not observed in
previous organs as with the liver.
In comparison to the spleen, Cr in the liver
increases with increased Mn as opposed to the
spleen where Cr was observed to decrease with
increased Mn. This observation may suggest a
relationship between increasing accumulation in the
liver and decreasing accumulation in the spleen. An
observation that has not been seen on previous
organs is with I. Iodine starts out with negative
accumulation at the lower Mn dosage. As Mn
dosage increases, Iodine fractional change becomes
Dietary Intakes Influence on Metallomic Distribution in Vital Organs and Their Implications
33
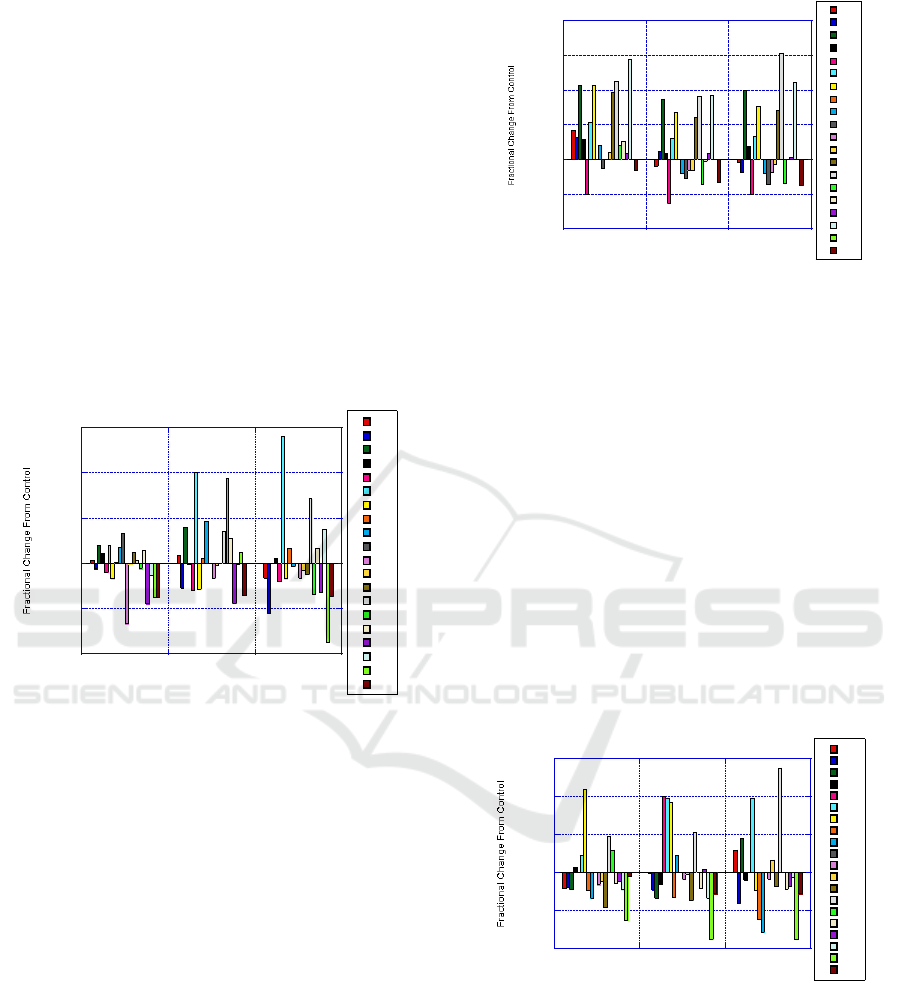
less. This suggests a mechanism that has the most
impact at lower dosage and then has less impact as
Mn dosages increase.
Another observation is that as Mn dosage
increases, more elements trend to a negative
accumulation. For unwanted or toxic elements, an
increased Mn dosage treatment reduces the
accumulation in the liver. As discussed with results
obtained with the kidney, the liver serves a similar
function in detoxification. This data supports that
increased Mn treatment provides for less
accumulation in the liver. There could be at least
two possible mechanisms. First is that increased Mn
treatment increases accumulation in other organs so
that the element is not available to accumulate in the
liver. A second possibility is that Mn treatment
improves the liver’s ability to filter out or flush away
unwanted elements.
-1
-0.5
0
0.5
1
1.5
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 5: Results of liver with Mn treatment for Group A,
B, and C as compared with control.
3.6 Pituitary
The pituitary shows an interesting trend that for each
treatment, the amount of change from control is
relatively the same. The same elements that show
increases show nearly the same increase for each
dosage treatment. Then for the elements that show a
decrease, maintain similar decreases cross the
dosage treatments.
In the pituitary, Mn treatment has a baseline
impact that is unrelated to Mn dosage level. The
presence of Mn has an impact but the concentration
of Mn dosage has little impact. . As overall more
elements have a negative impact for dosage
treatments in groups B and C compared to A. The
pituitary is not significantly impacted by Mn
treatment compared to other organs likely due to the
effect of the blood brain barrier.
-1
-0.5
0
0.5
1
1.5
2
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 6: Results of pituitary with Mn treatment for Group
A, B, and C as compared with control.
3.6 Uterus
The uterus shows more concentration decreases than
increases. Co, Cr and Cu show the most significant
increases. V, Fe and F show the most significant
decreases. For most elements, Mn decreases
accumulation. The large increases in heavy metals
(e.g. Co, Cr, and Cu) should be a signal for more
detailed investigation. The uterus is the key organ in
reproduction and significant accumulations can have
a detrimental impact on birth defects and maternally
associated diseases. As an overall observation,
increased Mn may be used to reduce elemental
accumulation in the uterus as well.
-1
-0.5
0
0.5
1
1.5
A B C
Al
Br
Ca
Cl
Co
Cr
Cu
F
Fe
Hg
I
K
Mg
Mn
Mo
Na
Rb
Se
V
Zn
Mn Treatment Dosage
Figure 7: Results of Uterus with Mn treatment for Group
A, B, and C as compared with control.
4 DISCUSSIONS
Each organ has different characteristics as indicated
by some showing overall accumulating
concentrations and some showing overall
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
34

diminishing concentrations, and others showing
mixed trends with increasing elemental
concentrations and decreasing elemental
concentrations. In a few instances, there were some
elements that showed a possible correlation with Mn
dosage. Both increasing concentrations with
increased Mn dosage and decreasing with increasing
Mn dosage were present suggesting positive and
negative correlations.
Three main observations can be concluded from
this analysis:
4.1 Elements Showing Increases
In most cases, treatment with Mn showed increased
elemental accumulation. Elements showing
accumulation in at least four organs are: Ca, Mg, Co,
Cr, Cu, Mo, and Se. In this group of elements, there
are some elements with known impacts on human
health and degenerative diseases (e.g., Cu related to
Alzheimer’s and Parkinson’s diseases). The
findings emphasize the importance of more
extensive studies where Mn may have been used in
applications that may have increased human
ingestion such as treatment of drinking water.
Elements showing accumulation in two or fewer
organs are: F, K, Na, Br, Hg, I, Rb, V, and Zn. Even
though accumulation was shown for this group of
elements, only certain organs displayed
accumulation. This is important to help identify
elements that may have more complicated
homeostatic mechanisms and are more selective to
individual organs. In this group, there also are
elements with known health impacts.
4.2 Elements Showing Increases
The liver and uterus show more overall decreased
elemental concentrations. The spleen and pituitary
also had several elements that decreased in
elemental concentration with Mn treatment.
Elements that show decreasing concentrations in
three or more organs are: Br, Hg, Se, V, and Zn.
Elements that show decreased concentration in one
organ are: Cl, Fe, K, Mg, Na, Al, Cr, Fe, I, and Rb.
Overall, more elements showed increased
concentrations or accumulation due to Mn treatment
than elements that showed decreased concentrations.
4.3 Overall Accumulating,
Diminishing, and Mixed
The organs that showed decreasing elemental
concentrations were the liver and uterus. The uterus,
showing a decrease in overall concentration, may
provide a link to the impact of Mn exposure and
possible birth defects. The liver, showing decreased
concentrations, may have two possible impacts.
First is the possibility that the elements are being
excreted from the body. This may be a positive
impact in regard to applications that would need to
reduce the concentration of a particular toxin in the
body. The second possible result in the liver
showing reduced elemental concentrations is that the
function of the liver is being reduced and therefore is
not pulling contaminants from the body.
5 CONCLUSIONS
From the literature search listed in this study, it is
evident there are many focused and detailed studies
showing the health impacts due to increased
elemental concentrations of particular elements.
One study identified Cu, Zn, Fe, and Mn are
essential for normal brain function, but also show
that above normal concentrations may lead to no
detectable (ND) symptoms. In this study, we found
the concentration of Cu is increasing in many organs
due to Mn treatments. In an evaluation of all brain
data, Cu decreases by 100% from adult control (AC)
to old control (OC) rats (Wright et al.).
The treatment of Mn could be beneficial in some
cases and detrimental in other cases. An increase in
Cu concentration (≈ 10%) in the brain may be
beneficial but an increase in Hg in the heart (≈
1500%) may be detrimental to humans. This data
set will provide a key piece in understanding human
health effects due to elevated elemental (i.e., heavy
metal) ingestion over the full life span.
The fact that there is hardly any information
available regarding elemental accumulation in
organs such as spleen, pituitary, and uterus makes
this massive collective study ever more valuable.
ACKNOWLEDGEMENTS
We want to acknowledge that Dr. Thomas K.C.
Leung, Institute of Molecular and Cell Biology,
Proteos, Singapore for his contributions in collecting
and organizing the data, and Professor Margret
Minsky, University of London for organizing and
conducting the elemental analyses.
REFERENCES
Anderson, J.G., Fordahl, S.C., Cooney, P.T., Weaver,
T.L., Colyer, C.L., Erikson, K.M., 2008, Manganese
exposure alters extracellular GABA, GABA receptor
Dietary Intakes Influence on Metallomic Distribution in Vital Organs and Their Implications
35

and transporter protein and mRNA levels in the
developing rat brain, Neurotoxicology, Vol. 29, pp.
1044-1053.
Bayer ,T.A., Schafer, S., Simons ,A., Kemmling, A.,
Kamer, T., Tepest, R., Eckert ,A., Schussel, K.,
Eikenberg, O., Sturchler-Pierrat ,C., Abramowski, D.,
Staufenbiel ,M., Multhaup, G., 2003, Dietary Cu
stabilizes brain superoxide dismutase 1 activity and
reduces amyloid Abeta production in APP23
transgenic mice, Proc Natl Acad Sci USA, Vol. 100,
pp. 14187–14192.
Bolognin, S, Messori, L, Zatta, P., 2009, Metal Ion
Physiopathology in Neurodegenerative Disorders,
Neuromoloecular Medicing, Vol. 11, pp. 223-238.
Maynard, C.J., Cappai, R., Volitakis, I., Laughton, K.M.,
Masters, C.L., Bush, A.I., Li, Q.X., 2009, Chronic
Exposure to High Levels of Zinc or Copper Has Little
Effect on Brain Metal Homeostasis or A beta
Accumulation in Transgenic APP-C100 Mice, Cellular
& Molecular Neurobiology, Vol. 29, pp. 757-767.
Pardridge, W., 2003, Blood-brain barrier drug targeting:
The future of brain drug development, Molecular
Interventions, Vol. 3, pp. 90–105. Pardridge, W., 2003,
Blood-brain barrier drug targeting: The future of brain
drug development, Molecular Interventions, Vol. 3,
pp. 90–105.
Wright, G., Lai, J., Chan, A., Minski, M., Leung, S., 2010,
Metallomic Distribution in Various Regions of the
Brain as Influenced by Dietary Intakes and
Implications, Procedia Environmental Sciences, Vol 2,
pp. 149-161.
(www.elsevier.com/locate/procedia)/(www.scien
cedirect.com)
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
36
