
Decolourization of Fast Red Acid dye using Photoactive Bi
2
O
3
Nanoparticle under Solar Irradiation
Yogendra Kambalagere
1*
, Madhusudhana. N
1
, Mahadevan. K. M
2
1
Department of P. G. Studies and Research in Environmental Science, Kuvempu University, Karnataka, India
2
Department of P. G. Studies and Research in Chemistry, Kuvempu University, Karnataka, India
Keywords: Bismuth Oxide (Bi
2
O
3
), Catalyst, Degradation, Fast Red acid dye, nanoparticle, photodegradation, SEM,
XRD
Abstract: The photocatalytic efficiency of prepared Bismuth Oxide (Bi
2
O
3
) nano catalyst was investigated against
colour induced by Fast Red acid dye. The Bi
2
O
3
nanoparticles were prepared by a simple solution
combustion method and the average powder size of the obtained Bi
2
O
3
nanoparticles was determined by
SEM and XRD analysis. The research work was done to determine the influencing parameters like,
optimum catalyst dosage, pH of the dye solution, effect of sunlight irradiation and effect of dye
concentration. The treatment with prepared Bi
2
O
3
nanoparticle proved to be very efficient in removing the
colour (~ 95.02%) at pH 10 and 0.8g/100ml of catalyst dose. The Bi
2
O
3
nanoparticle was found to be an
effective and low cost photocatalyst by degrading Fast Red acid dye in presence of sunlight.
1 INTRODUCTION
Over the decades, an enormous interest has been
developed over photocatalysis using oxide
semiconductors in environmental applications.
Textile processing industries are widespread and
major industrial sectors in developing countries.
From the most used processes in the textile industry,
dyeing process consumes most of the available water
for dyeing, fixing, and washing processes. The
textile industry utilizes about 10000 different dyes
and the worldwide annual production of dyestuffs is
more than 7×105 tons (2013).
Among several textile dyestuffs, the reactive
dyes contribute about 50% (2007) of the total market
share and the most common group used as
chromophore is the azo dye (70%), followed by
anthraquinone (2004). It has been a known fact and
also a documentation that dye loss in wastewaters
could vary up to 50% during manufacturing or
processing operations. The release of the coloured
wastewaters to the water bodies will bring a drastic
change in the form of pollution and results in the
formation of toxic and mostly non-biodegradable
substances in the ecosystem. The colouration is
visible even in a very low dye concentration (below
1mgL
−1
) (2004). As a result, technological systems
for the removal of organic pollutants such as
adsorption on active carbon, reverse osmosis, ion
exchange on synthetic adsorbent resins, ozonation,
and biological methods were examined (2001). All
of them have advantages and drawbacks but these
processes have very high operating costs. However,
these conventional treatment methods are not so
efficient in removing the dyes from effluents, so
finding an alternative and effective technique is
necessary (2004), (2015), (2002), (2017).
A watchful time has been spent to develop dye
treatment methods at its source (ISO 14001, October
1996). An alternative to conventional methods, such
as, “advanced oxidation process” (AOP) has been
developed based on the generation of very reactive
species such as hydroxyl radicals. The generated
hydroxyl radicals can oxidize a wide range of
organic pollutants quickly and non-selectively.
Among the (AOPs), heterogeneous photocatalysis
appears as an emerging solution to the
environmental pollution for aquatic system. This
process consists of the non selective destruction of
organic compounds in presence of natural light and
photocatalysis systems such as TiO
2
, ZnO, and CdS
(2015).
In this study we have synthesized bismuth oxide
nanoparticles by simple solution combustion method
using stoichiometric equations. Bismuth oxide is an
Kambalagere, Y., M, M. and K M, M.
Decolourization of Fast Red Acid dye using Photoactive Bi2O3 Nanoparticle under Solar Irradiation.
DOI: 10.5220/0008652200110016
In Proceedings of the International Conference on Future Environment Pollution and Prevention (ICFEPP 2019), pages 11-16
ISBN: 978-989-758-394-0
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
11
important metal-oxide semiconductor, having an
excellent optical and electrical property such as wide
band-gap, high refractive index and
photoconductivity.
Therefore, it has been used widely in many fields
such as solid oxide fuel cells, gas sensors,
photoelectric materials, high temperature
superconductor materials, catalysts and functional
ceramics (2013). For the above reasons the objective
of this study was to explore the possibility
photocatalytic degradation on fast red dye by
varying the different parameters such as, initial dye
concentration, pH, catalyst loading and in different
conditions with respect to UV light and dark
conditions.
2 MATERIALS AND METHODS
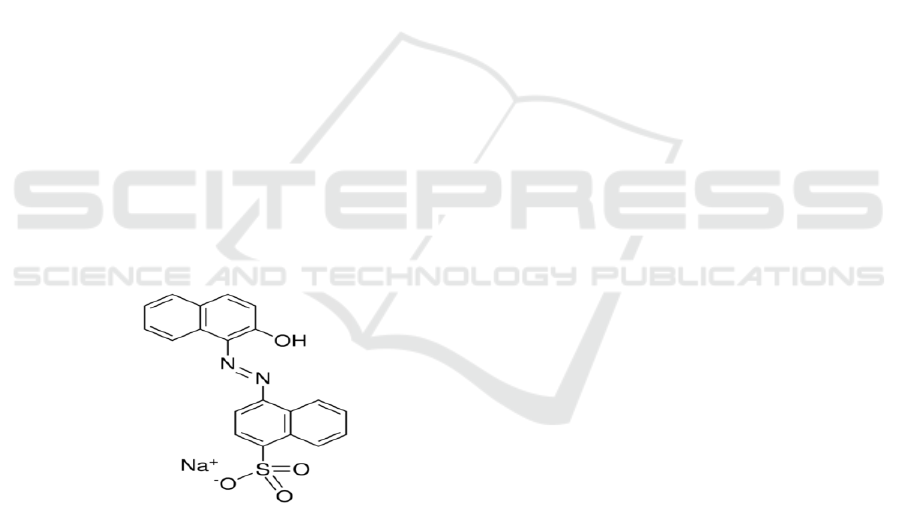
The commercially available water soluble dye fast
red (λ
max
525nm) was obtained from Sisco Research
Laboratory Pvt. Ltd. Maharashtra (Figure 1). The
chemicals Bismuth Nitrate (Bi(NO
3
)
3
5H
2
O) obtained
from Sisco Research Laboratory Pvt. Ltd.
Maharastra and Urea (NH
2
CONH
2
) obtained from
Hi-Media Chemicals, Mumbai. The Visible
spectrophotometer (Elico, SL 177) was usedfor
recording absorbance at λ
max
. Later the absorbance
was recorded in visible spectrophotometer (Elico,
SL 177).
Figure 1: Structure of Fast Red Dye
2.1 Synthesis and Characterization of
nanoparticles
6 Bi(NO
3
)
3
+ 15 NH
2
CONH
2
→ 3 Bi
2
O
3
+ 15 CO
2
+ 30 H
2
O + 24 N
2
(Eq.1)
The solution taken in a crucible was kept in a
preheated muffle furnace at 600°C and the
synthesized nanoparticles was crushed in a mortar to
make the powder amorphous and used for further
characterization. Characterization of nanoparticles
by Powder X-ray diffraction (XRD) was performed
by using Rigaku diffractometer by Cu-Kα radiation
(1.5406 Å) in a θ-2θ configuration. Scanning
electron microscope (SEM) image was taken with a
JEOL (JSM-840A). The UV-visible spectra of the
photocatalysts were carried out using a UV-visible
spectrophotometer in the λ range from 200 to 1200
nm. The confirmatory presence of elements was
carried out using Energy Dispersive X-ray (EDX)
spectrometer.
2.1.1 Experimental Procedure
Photocatalytic experiments were carried out under
direct sunlight. The fast red solution was prepared
by dissolving 0.02 g of dye with 1000mL double
distilled water using a 1000ml volumetric flask and
degradation in the presence of bismuth oxide
nanoparticles at different catalyst dosages pH levels
and initial dye concentration. Initially, 100ml of
20ppm dye samples were tested with different
catalyst dosages (from 0.1g to 1g), by varying
pH(from 2pH to 11pH), dye concentration (20ppm
to 40ppm) and different conditions with respect to
U.V and dark. Except U.V and dark conditions all
experiments were carried out in presence of direct
sunlight. The whole experimental set-up was placed
under sunlight between 10 am to 2 p.m. and the
average intensity of sunlight during this period was
found to be 100000 to 130000 lux. After the
photocatalytic degradation, the degradation
percentage was estimated by recording absorbance
of the dye solution using spectrophotometer (Elico,
SL 177) in order to get the optimum catalyst dose.
The percentage was calculated using the equation,
D = (A
0
- A
t
/ A
0
× 100) (Eq. 2)
A
0
is initial concentration of fast red dye and A
t
is the concentration of Fast Red dye at time ‘t’
2.2 Result and Discussion
Characterization of the Nanoparticles
2.2.1 X-ray Diffraction [XRD]
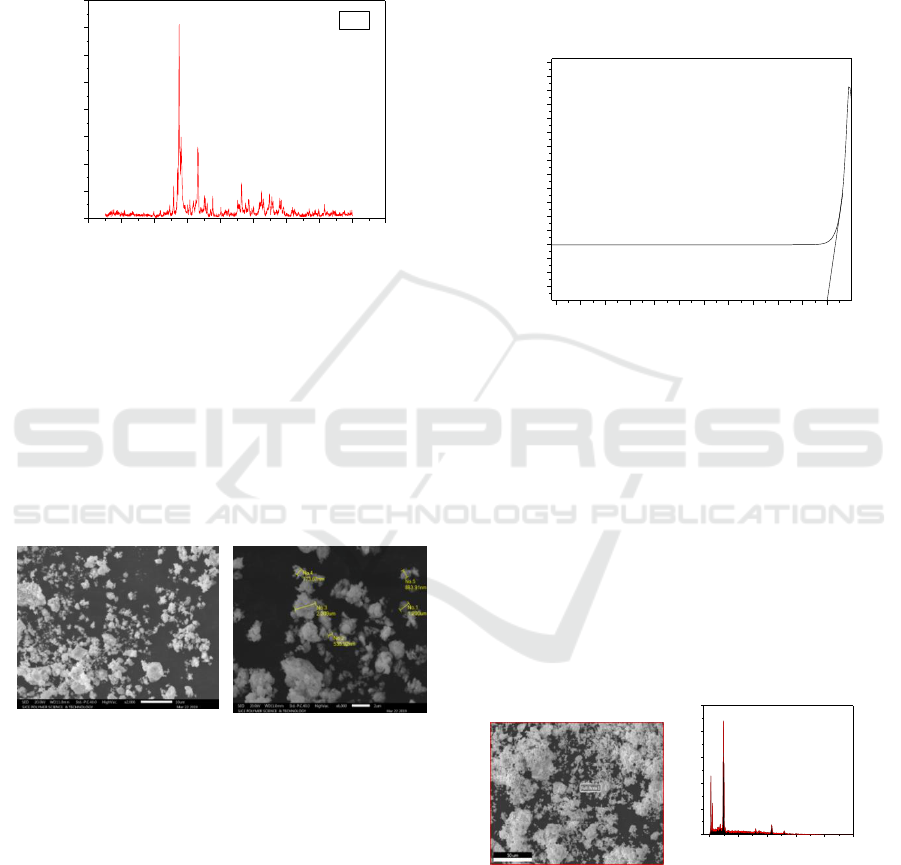
The pattern obtained from the XRD analysis of the
prepared bismuth oxide nanoparticles is shown in
figure 2. According to the Debye Scherrer’s
formula:
D = Kλ/β Cosθ (Eq. 3)
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
12
Where K is the Scherrer’s constant, λ the X-ray
wavelength, β is the peak width at half-maximum
and θ is the Bragg’s diffraction angle.
In the present work, the powdered sample of
bismuth oxide by XRD studies found that, the size
was varied from 20 nm to 55 nm and its average size
was achieved at 36 nm respectively.
Figure 2: XRD of the synthesized Bi
2
O
3
nanoparticles
2.2.2 Scanning Electron Micrograph [SEM]
Scanning Electron Microscope pictures depicts
bismuth oxide nanoparticles. The photograph depicts
an aggregated, cluster, foamy in nature. The
enlarged image shows the agglomeration, uneven
texture of the different nanoparticles and also shows
strong bonding of nanoparticles over one another
(Fig. 3).
Figure 3: Scanning Electron Micrographs of synthesized
Bi
2
O
3
Nanoparticles
2.2.3 UV-Vis Spectroscopy
The optical absorption is a significant tool to
get optical energy band gap of crystalline and
amorphous materials. The elemental absorption
corresponds to the electron jump from valence band
to the conductivity band. The spectrum reveals that,
the Bismuth oxide nanoparticle absorption in the
visible radiation with an above wavelength 400 nm.
The value of optical band gap (OBG) is calculated
from the TAUC’s relation:
[αhυ]=B[hυ−Eg]
n
(Eq. 4)
Where, ‘hυ’ is the photon energy, ‘B’ is the
constant and ‘n’ is the power factor and that takes
1/2, 2, 3/2 and 3 allowed direct, allowed indirect,
forbidden direct and forbidden indirect transitions
respectively. The OBG of the bismuth oxide
nanoparticle found to be 3.6eV.
Figure 4: UV-absorption spectra of synthesized Bi
2
O
3
nanoparticles
2.2.4 Energy Dispersive X-ray
The confirmatory presence of elements was carried
out using Energy Dispersive X-ray [EDX]
spectrometer. The presence of bismuth, Carbon and
Oxygen signals from the Bismuth oxide
nanoparticles (Fig. 5). The weight and atomic
percentage of Carbon, Oxygen, and Bismuth was
found to be 22.82, 16.23, 60.95 and 58.26, 31.11,
10.63 these corresponds, the spectrum without
impurities peaks.
Figure 5: Energy Dispersive X-ray of synthesized Bi
2
O
3
nanoparticles
2.2.5 Effect of Catalyst Concentration
The effect of catalyst concentration on the
photocatalytic degradation was studied at the range
of catalyst amount from 0.1 to 1g/100ml for fast red
0 10 20 30 40 50 60 70 80 90
0
200
400
600
800
1000
1200
1400
1600
INTENSITY
2
Bi
2
O
3
2.5 2.6 2.7 2.8 2.9 3.0 3.1 3.2 3.3 3.4 3.5 3.6
-6.00E-070
-4.00E-070
-2.00E-070
0.00E+000
2.00E-070
4.00E-070
6.00E-070
8.00E-070
1.00E-069
1.20E-069
1.40E-069
1.60E-069
1.80E-069
2.00E-069
2.20E-069
2.40E-069
2.60E-069
(
h
)
eV
0 5 10 15 20 25
0
300
600
900
1200
1500
O
C
Bi
Bi
Decolourization of Fast Red Acid dye using Photoactive Bi2O3 Nanoparticle under Solar Irradiation
13
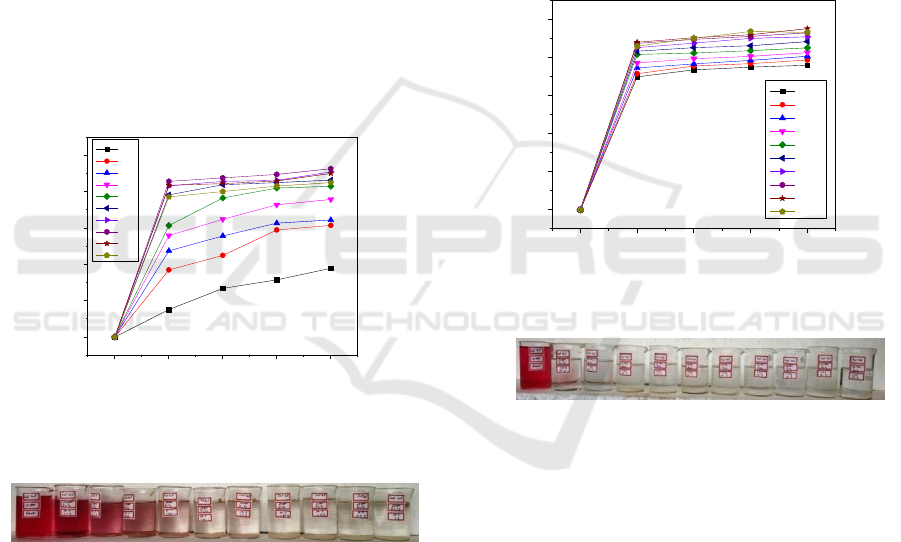
dye. The synthesized nanoparticle shows appreciable
results. The bismuth oxide (urea) with the
nanoparticle size 36 nm has shown 92.62 %
degradation. Since, the photodegradation was very
efficient at 0.8g/100ml in 120 minutes for Bi
2
O
3
nanoparticles concentration showed in (Figure-6)
(Photo-1).
The increase in degradation rate can be
determined in terms of availability of active sites on
the catalyst surface and sunlight penetration into the
suspension as a result of increased screening effect
and scattering of light. A further increase in the
catalyst amount beyond the optimum dosage for the
obtained nanoparticles decreases the
photodegradation by some margin. This may be due
to overlapping of adsorption sites as a result of
overcrowding owing to collision with ground state
catalyst (2018), (2007). Since, the photodegradation
was very effective at 92.62% 0.8g/100ml for Bi
2
O
3
nanoparticle dosages, further experiments were
continued with the obtained dosage.
Figure 6: Effect of catalyst concentration on dye solution
at 120 minutes=20 ppm, pH=7
Photo 1. Effect of catalyst concentration on Fast Red dye
at 120 minutes=20 ppm, pH=7
2.2.6 Effect of pH
To study the effect of pH on the degradation
efficiency of Bi
2
O
3
catalyst, the experiments were
carried out at pH ranging from 2 to11. The results
showed that pH significantly affected the
degradation efficiency. The percentage of
degradation of fast red for Bi
2
O
3
(Fig.7) (Photo 2)
nanoparticles was achieved at 76.11% to 95.32%
from pH 2 to 10, similarly, the degradation
decreases to 93.33% at pH 11 in 120 minutes for
0.8g/100ml. The maximum degradation was found
at pH 10. The results from the experiment show that,
the degradation was effectively in pH 10 due to the
interaction between the dye and nanoparticles leads
to generation of OH
•
in the alkaline medium which
are responsible for the photodegradation. Above pH
10 the degradation decreases due to amphoteric
nature of the catalyst and electrostatic repulsion
between negatively charged dye molecules and the
catalyst (Journal of Photochemistry and
Photobiology A: Chemistry.vol. 158, no. 1, pp. 27-
36), (2012), (2016), (Solar Energy Materials &
Solar Cells.vol. 77, no. 1, pp. 65–82.). Thus, the
adsorption is mainly depends on the pH of the
solution.
Figure 7: Effect of pH on dye at 120 minutes
Photo. 2. Effect of pH on dye at 120 minutes
2.2.7 Effect of Initial Dye Concentration
For effect of (Fast Red) initial dye concentration, the
dye solution concentration was varied from 20 ppm
to 40 ppm. The results obtained for Bi
2
O
3
(Fig. 8)
(Photo 3) is 95.32% for 20ppm, 78.70% for 30ppm
and 54% for 40ppm, these experiments illustrated
that the degradation efficiency was directly affected
by the concentration. The decrease in the
degradation with an increase in dye concentration
was ascribed to the equilibrium adsorption of dye on
the catalyst surface which results in a decrease in the
active sites. This phenomenon results in the lower
formation of OH· radicals which were considered as
primary oxidizing agents of the organic dye (2012),
(Journal of Iranian Chemical Society.vol. 6, no. 3,
pp. 578-587). According to Beer Lambert’s law, as
the initial dye concentration increases, the path
Blank 30Min 60Min 90Min 120Min
0
20
40
60
80
100
Percentage of Degradation
Time/ Min
0.1g
0.2g
0.3g
0.4g
0.5g
0.6g
0.7g
0.8g
0.9g
01g
Blank 30 Min 60 Min 90 Min 120 Min
0
20
40
60
80
100
Percentage of Degradation
Time/Min
pH 2
pH 3
pH 4
pH 5
pH 6
pH 7
pH 8
pH 9
pH 10
pH 11
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
14

length of photons entering the solution decreases.
This results in the lower photon adsorption of the
catalyst particles, and consequently decreases
photocatalytic reaction rate (2006).
Figure 8: Effect of initial dye concentration on the
photocatalytic degradation of Fast Red dye, Bi
2
O
3
g/pH=0.8/10
Photo. 3. Effect of initial dye concentration on the
photocatalytic degradation of Fast Red dye, Bi
2
O
3
g/pH=0.8/10
2.2.8 Effect of Sunlight Irradiation on Fast
Red Dye
Figure 9: Effect of sunlight irradiation with respect to
Dark condition and UV condition on photocatalytic
degradation of Fast Red dye in 120 minutes
Photo. 4. Effect of sunlight irradiation with respect to
Dark condition and UV condition on photocatalytic
degradation of Fast Red dye, in 120 minutes
The photocatalytic degradation of fast red dye
(20mg/L) under three different experimental
conditions were examined, i.e., through
dye/dark/catalyst, dye/UV/catalyst and
dye/sunlight/catalyst. Fast red dye solution when
exposed directly to the sunlight without the catalyst,
the degradation was found to be zero during the
entire experiments. The degradation rate was found
to be increased with increase in irradiation time, for
dye/sunlight/Bi
2
O
3
showed 95.32%, dye/UV/Bi
2
O
3
found to be 76.34% and for dye/dark/Bi
2
O
3
22.25%
was recorded (Fig. 9). The obtained results show
that photodegradation occurs most efficiently in the
presence of sunlight (Photo 4). Under sunlight,
excitation of electrons from the catalyst surface
takes place more rapidly than in the absence of light
(2010), (2009).
4 CONCLUSIONS
In the present study, solar photocatalytic degradation
of textile dye, Fast Red dye has been investigated by
using synthesized Bi
2
O
3
nanoparticles and found
that, Bi
2
O
3
nanoparticles synthesized economically,
conveniently and quickly with the available cost
effective metal nitrates. At lower catalyst
concentration, the catalyst surface and adsorption of
dye on the catalyst surface are the limiting factors.
Thus, an increase in catalyst concentration greatly
enhances the efficiency of the process. On the other
hand, at very high concentration, overlapping of
adsorption site and deactivation of activated catalyst
reduces the process efficiency. The degradation
efficiency increased with an increase in pH,
attaining maximum decolourization at pH 10.
In the present study, it is found that synthesized
Bi
2
O
3
nanoparticles exhibit excellent photocatalytic
activity against Fast Red dye and can be used in
water purification systems and dye effluent
treatment.
REFERENCES
Khezrianjoo, S., Revanasiddappa, H.D., 2013.
Photocatalytic Degradation of Acid Yellow 36 Using
Zinc Oxide Photocatalyst in Aqueous Media. Journal
of Catalysts. vol. 1, no. 1, pp.1-6.
Blank 30 Min 60 Min 90 Min 120 Min
0
20
40
60
80
100
Percentage of Degrdation
Time/Min
20 ppm
30 ppm
40 ppm
Blank 30 Min 60 Min 90 Min 120 Min
0
20
40
60
80
100
Percentage of Degradation
Time/Min
Sunlight
UV Light
Dark Condition
Decolourization of Fast Red Acid dye using Photoactive Bi2O3 Nanoparticle under Solar Irradiation
15

Sleiman, M., Vildozo, D., Ferronato, C., Chovelon, J.M.,
2007. Photocatalytic degradation of azo dye
metanilyellow optimization and kinetic modeling
using a chemometric approach. Applied Catalysis B.
vol. 77, no. 1-2, pp. 1–11.
Lee, Y.H., Pavlostathis, S.G., 2004. Decolorization and
toxicity of reactive anthraquinone textile dyes under
methanogenic conditions. Water Research., vol. 38,
no. 7, pp. 1838–1852.
Konstantinou, I.K., Albanis, T. A., 2004. TiO
2
-assisted
photocatalytic degradation of azo dyes in aqueous
solution: kinetic and mechanistic investigations: a
review. Applied Catalysis B. vol. 49, no. 1, pp. 1–14.
Galindo, C., Jacques, P., Kalt, A., 2001. Photooxidation of
the phenylazonaphthol AO
2
0 on TiO
2
: kinetic and
mechanistic investigations. Chemosphere. vol. 45, no.
6-7, pp. 997–1005.
Chakrabarti, S., Dutta, B.K., 2004. Photocatalytic
degradation of model textile dyes in wastewater using
ZnO as semiconductor catalyst. Journal of Hazardous
Materials. vol. 112, no. 3, pp. 269-278.
Mohabansi, N.P., Satone, A.K., 2015. Solar Photocatalytic
Degradation of Textile Effluents by Using Titanium
Dioxide. International Journal of Pharmaceutical,
Chemical & Biological Sciences.vol. 5, no. 2, pp. 487–
490.
Neppolian, B., Choi, H.C., Sakthivel, S., Arabindoo, B.,
Murugesan, V., 2002. Solar light induced and TiO
2
assisted degradation of textile dye reactive blue 4.
Chemosphere, vol. 46, no. 1, pp. 1173-1181.
Santhosh, A.M., Yogendra, K., Mahadevan, K.M.,
Madhusudhana, N., 2017. Photodegradation of Congo
red azo dye, a Carcinogenic Textile dye by using
synthesized Nickel Calciate Nanoparticles.
International Journal of Advance Research in Science
and Engineering. vol. 6, no. 7, pp. 51-64.
Madhusudhana, N., Yogendra, K., Mahadevan, K.M.,
2015. A study on photocatalytic decolourization of
Violet GL2B azo dye using two different synthesized
nano particles CaZnO
2
against commercially available
TiO
2
. International Journal of Chemical and
Environmental Engineering. vol. 6, no. 4, pp. 210-218.
Khanmohammadi, H., Rezaeian, D.K., 2015. Solar-light
induced photodecolorization of water soluble azo-
azomethine dye : influence of operational parameters
and nanophotocatalysts. Desalination and Water
Treatment.vol. 55, no. 3, pp. 655-663.
La, J., Huang, Y., Luo, G., Lai, J., Liu, C., Chu, G., 2013.
Synthesis of Bismuth Oxide Nanoparticles by Solution
Combustion Method. Particulate Science and
Technology.Vol. 31, no. 1, pp. 287-290.
Madhusudhana, N., Yogendra, K., Mahadevan, K.M.,
Santhosh, A.M., 2018. Study on Photocatalytic
Degradation Coralene Red F3BS by using Synthesized
Al
2
O
3
Nanoparticles. AGU International Journal of
Engineering and Technology.vol. 6, no. 1, pp. 29-37.
Subramani, A.K., Byrappa, K., Ananda, S., Lokanatha
Rai, K.M., Ranganathaiah, C., Yoshimura, M., 2007.
Photocatalytic degradation of indigo carmine dye
using TiO
2
impregnated activated carbon. Bulletin of
materials science.vol. 30, no. 1, pp. 37-41.
Guillard, C., Lachheb, H., Houas, A., Ksibi, M., Elaloui
E., Herrman, J.M., 2003. Influence of chemical
structure of dyes of pH and of inorganic salts on their
phtocatlytic degradation by TiO
2
Comparison of
efficiency of powder and supported TiO
2
. Journal of
Photochemistry and Photobiology A: Chemistry.vol.
158, no. 1, pp. 27-36.
Mehta, R., Surana, M., 2012. Comparative study of photo-
degradation of dye Acid Orange-8 by Fenton reagent
and Titanium Oxide- A review. Der Pharma
Chemica.vol. 4, no. 1, pp. 311-319.
Sobana, N., Thirumalai, K., Swaminathan, M., 2016.
Kinetics of Solar Light Assisted Degradation of Direct
Red 23 on Activated Carbon-loaded Zinc Oxide and
Influence of Operational Parameters. Canadian
Chemical transactions.vol. 4, no. 1, pp. 77-89.
Sakthivel, S., Neppolian, B., Shankar, M.V., Arabindoo,
B., Palanichamy, M., Murugesan, V., 2003. Solar
photocatalytic degradation of azo dye: comparison of
photocatalytic efficiency of ZnO and TiO
2
. Solar
Energy Materials & Solar Cells.vol. 77, no. 1, pp. 65–
82.
Gopalappa, H., Yogendra, K., Mahadevan, K.M.,
Madhusudhana, N., 2012. Solar Photocatalytic
Degradation of Orange G (Mono azo dye) and C.I.
Direct Yellow 50 (Di azo dye) by Synthesized
CaZnO
2
Nanoparticle in aqueous solution.
International Journal of Universal Pharmacy and Life
Sciences.vol. 2, no. 4, pp. 66-77.
Mirkhani, V., Tangestaninejad, S., Moghadam, M.,
Habibi, M.H., Rostami, V.A., 2009. Photocatalytic
Degradation of Azo dyes Catalyzed by Ag Doped
TiO
2
Photocatalyst. Journal of Iranian Chemical
Society.vol. 6, no. 3, pp. 578-587.
Byrappa, K., Subramani, A.K., Ananda, S., LokanathaRai,
K.M., Dinesh, R., Yoshimura, M., 2006.
Photocatalytic degradation of Rhodamine B dye using
hydrothermally synthesized ZnO. Bulletin of Material
Sciences.vol. 29, no. 5, pp. 433-438.
Guillen, S.A., Mayen, S.A., Torres-Delgado, G.,
Castanedo-Perez, R., Maldonado, A., De la Olvera,
M.L., 2010. Photocatalytic degradation of methylene
blue using undoped and Ag doped TiO
2
thin films
deposited by a sol gel process: Effect of the ageing
time of the starting solution and the film thickness.
Materials Science and Engineering: B.vol. 174, no. 1-
3, pp. 84-87.
Movahedi, M., Mahjoub, A.R., Janitabar-Darzi, S., 2009.
Photodegradation of Congo Red in Aqueous Solution
on ZnO as an Alternative Catalyst to TiO
2
. Journal of
the Iranian Chemical Society.vol. 6, no. 3, pp. 570-
577.
ICFEPP 2019 - International conference on Future Environment Pollution and Prevention
16
