
Accumulation of Heavy Metals of Cooper (Cu) and Lead (Pb) on
Rhizophora mucronata in Mangrove Forest, Nelayan Village Sub
Medan Labuhan Subdistrict and Jaring Halus Village, Secanggang
Subdistrict, North Sumatra, Indonesia
Yunasfi
1
, Kanvel Prit Singh
1
and Desrita
2
1
Faculty of Forestry, University of Sumatera Utara, Jl. Tri Dharma No. 1, Campus USU Medan 20155
2
Faculty of Agriculture, University of Sumatera Utara, Jl. A. Sofyan No.1 Medan 20155
Keywords: Heavy Metal, Cooper (Cu), Lead (Pb), AAS, R. mucronata
Abstract: This study aimed to analyzes the content of heavy metals Cu and Pb in the roots, leaves and barks of R.
mucronata and analyze the ability of R. mucronata in accumulating heavy metals. Sampling was carried out
at Medan Labuhan Sub-District Nelayan Village and Jaring Halus Village, Secanggang District. The analysis
of Cu and Pb heavy metals was carried out at the Research Laboratory, Faculty of Pharmacy, University of
North Sumatera. Using the Atomic Absorption Spectrophotometer (AAS) method.The results of this study
indicate that the heavy metal content of Cu on the barks and leaves in Jaring Halus Village are higher than
Nelayan Village. The content of Cu in roots are higher in Nelayan Village. The content of Pb Metal in the
barks and roots in Jaring Halus Village are higher than Nelayan Village. The content of Pb metal in the
leaves are higher in the Nelayan Village. Based on bio concentration factors, R. mucronata's ability to
accumulate Cu heavy metals are categorized as medium, whereas in accumulating Pb heavy metals are
categorized as low.
1 INTRODUCTION
1.1 Background
Mangroves are one of several coastal ecosystems
that have an important role. Mangrove ecosystems
have the highest level of productivity compared to
other coastal ecosystems. The large number o f
businesses using mangroves have caused the area of
mangrove decreasing from year to year. These
activities include coastal reclamation, land clearing
for agriculture and aquaculture, industry and housing
development in coastal areas. The direct impact
caused by above activities is the entry of waste into
the mangrove ecosystem, paricullary the waste
which contains heavy metals (Hamzah and
Setiawan, 2010). Mangrove community often gets
supplies of pollutants such as heavy metals from
industrial, household and agricultural waste.
Mangrove plants include types of aquatic plants that
have a very high ability to accumulate heavy metals
in the waters. The process of absorbtion in plants
occurs as various diffusion processes and the term
used is translocation in animals. This transport
occurs from cell to cell to vascular tissue so that it
can be distributed to all parts of the body.
Soemirat (2003) states that the absorption
process can occur through several parts of plants,
namely:
1 Roots, especially for inorganic substances and
hydrophilic substances.
2 Leaves for substances that are lipophilic.
Based on Panjaitan's, et al., (2009) data obtained
on the content of heavy metals Pb and Cu in the
mangrove forest of the Nelayan Village in Medan
Labuhan District. In the water, obtained Cu content
of 0.1198 mg / L and Pb content of 0.4522 mg / L.
In sediments, obtained Cu content of 9.0735 mg / L
and PB content of 9.9500 mg / L. From the data, it
was found that sea water in the Mangrove Forest of
Medan Labuhan Subdistrict was contaminated with
heavy metals Cu and Pb because it exceeded the
limit set by the LPM No.51 of 2004 KEPMEN,
which was 0.05 mg / L.
Based on Handayani's research, (2006) data
obtained on accumulation of Cu heavy metals at the
root of R. mucronata trees amounted to 24.431 ppm.
Yunasfi, ., Singh, K. and Desrita, .
Accumulation of Heavy Metals of Cooper (Cu) and Lead (Pb) on Rhizophora mucronata in Mangrove Forest, Nelayan Village Sub Medan Labuhan Subdistrict and Jaring Halus Village,
Secanggang Subdistrict, No.
DOI: 10.5220/0008552402410247
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 241-247
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
241

This shows that R. mucronata trees can be used as
bioaccumulators of Cu heavy metals in mangrove
forests.
1.2 Research Purpose
1. Determine the heavy metals content of Cu and
Pb in the roots, leaves and barks of the tree
Rhizophora mucronata.
2. Analyze R. mucronata's ability to accumulate
heavy metals Cu and Pb in the Mangrove Forest
of Jaring Halus Village, Secanggang District and
in the Nelayan Village of Medan Labuhan Sub-
Village, so that it can be used as an accumulator
of heavy metal pollution in the mangrove forest
area.
2 MATERIAL AND METHOD
2.1 Time and Location
This research was conducted in two observation
stations, station I was in the coastal area of Belawan,
namely Mangrove Forest of Nelayan Village as an
area suspected of being polluted because it was close
to industry and station II in Mangrove Forest Jaring
Halus Village which was suspected of being
uncontaminated (control) because it was far from the
industry. Heavy metal analysis was carried out at the
Research Laboratory, Faculty of Pharmacy
Universitas Sumatra Utara from April to August
2018.
2.2 Materials
The equipment used in this study consists of:
knife, measuring tape, camera, compass, mortal and
pastle, aquadest bottle, 250 ml Erlenmeyer flask,
drip pipette, furnace (furnace), oven, funnel,
Whatman filter paper size 42, universal pH,
porcelain crucible, measuring cup, beaker glass, 100
ml and 25 ml measuring flask, thermometer, hand
refractometer, hot plate, sample container, analytical
scale, and atomic absorption spectrophotometry.
The materials used for this research are:
sampling tally sheet, raffia, concentrated HNO3
solution, aqua bides, standard Cu and Pb solution, R.
muconata roots sample consist of taproot, R.
muconata leaves consist of old leaves and young
leaves, R. mucronata barks affected by tides,
sediment samples and seawater samples.
2.3 Sampling Procedure
Sampling in both locations were carried out by
purposively following the transect path along the
coastline. The roots, leaves, and barks samples were
taken from R. muconata tree. Roots taken are
taproots that are above the limit exposed to the tidal
boundaries of the sea, while for the leaves taken are
young leaves on the shoots and old leaves at the base
of the twigs, the barks of the R. mucronata tree
taken are tidal bark sea water. From the transect
path, 3 sample points were taken at each location
with a sample distance of 50 meters. Sample taken at
each point with three replications. Data taken in the
form of roots, leaves, and stem barks of R.
mucronata. As supporting data, measurements of
heavy metals in surface and sediment water (± 30
cm depth) as well as measurements of water quality
parameters, such as air temperature, water
temperature, water pH, and salinity at the six points
were also measured.
2.4 Preparation of Roots, Leaves,
Barks and Sediment Samples
Roots, leaves and barks samples are homogenized
by compiling samples taken from three extraction
points at each station. For the preparation of roots,
leaves, and barks, the samples are cut into small
pieces before smoothing. Likewise, sediment
samples can be smoothed directly. After that they sre
dried in an oven at a temperature of 105º C until a
constant weight is obtained.
Samples of roots, leaves, barks and sediments are
each weighed as much as 5 grams, placed on a hot
plate to become charcoal. To speed up the
occurrence of charcoal, a small amount of HNO3
can be dripped slowly. Samples that have become
charcoal are added to the furnace at a temperature of
700º C (ignition) until become ash. After the ash
process, roots, leaves and sediment samples
dissolved by adding 10 ml of concentrated HNO3.
The solution mixture was crushed in porcelain
crucible and then filtered into a 25 ml volumetric
flask using whatman filter paper size 42. Crushed
crisps were rinsed using twice aqua bides so that the
metal content still attached to the crucible dissolved.
After the filter is filtered, add aqua bides to the
boundary line on the measuring flask. The solution
obtained can be tested using AAS.
ICONART 2019 - International Conference on Natural Resources and Technology
242

2.5 Water Sample Preparation
Seawater measured 100 ml, then added 10 ml of
concentrated HNO3. Heat in an Erlenmeyer
container on a hot plate until the volume becomes 30
ml. Drop the aqua bides solution until the volume
becomes 100 ml then deposited. The precipitated
solution is filtered by the water phase with whatman
filter paper size 42. The solution obtained is ready to
be analyzed using AAS.
2.6 Principle of Atomic Absorpsion
Spectrophotometer (AAS)
AAS is set in advance according to the instructions
in the manual tool. Then calibrated with a standard
curve of each Cu and Pb metal with a concentration
of 0; 0.2; 0.4; 0.6; 0.8 and 1 ppm. The absorbance
and concentration of each sample were measured.
2.7 Data analysis
2.7.1 Real Concentration
To get the actual heavy metal concentration on the
roots, barks, leaves and sediments in accordance
with the standard operating procedures at the
Research Laboratory, Faculty of Pharmacy,
Universitas Sumater Utara, the formula used is:
Real consentration (mg/L) =
Consentration AAS (mg/l) x Solvent volume (l)
(1)
Sample weight (mg)
To get the actual concentration of heavy metals in
water, the formula used is:
Real consentration (mg/L) =
Consentration AAS (mg/l) x Sample solution (l)
(2)
Sample weight (mg)
2.7.2 Bio concentration factor (BCF)
After the heavy metals content in the water have
known, the data is used to calculate the ability of R.
mucronata to accumulate heavy metals Cu and Pb
through the level of bio concentration factor (BCF)
using the formula:
BCF Cu / Pb =
(Heavy metal Cu / Pb) Plant)
(3)
(Heavy metal Cu / Pb) Water
Information :
BCF> 1000 = High Ability
1000> BCF> 250 = Moderate Ability
BCF <250 = Low Ability
2.8 Descriptive Analysis
The data obtained was analyzed descriptively
according to the environmental quality standards
mentioned in the Decree of the Ministry of
Environment No. 51 of 2004 for water quality.
Quality standard for heavy metals in mud or
sediment in Indonesia have not yet been established,
so that as a reference, IADC / CEDA (1997) issued
quality standards regarding metal content that can be
tolerated.
3 RESULT
3.1 Aquatic Environment Conditions
(temperature, water temperature,
water pH, and salinity)
The condition of the aquatic environment results
from in-situ measurements in the field, showing the
different results between observation points. The
temperature and the highest water temperature are
found in the Mangrove Forest of Jaring Halus
Village as well as the pH of the water. The highest
salinity was obtained in the Mangrove Forest of the
Nelayan Village (Table1).
Temperature at station II is higher than
temperature at station I. This can be caused by the
geographical location of the two observation
stations. Temperature of the two observation stations
can be categorized as high, this can occur due to the
high intensity of the sun during sampling process.
Water temperature at station I is lower because
the presence of R. mucronata trees in the Mangrove
Forest of Nelayan Village is in a fairly close water
surface closure. Whereas at the second station the
existence of the R.mucronata tree is at the edge of
the bay so that the closure of the water surface by
the canopy is quite tenuous.
From the results of salinity measurements at both
stations, the salinity range at station I is around 20-
30 ppt with an average value of 23.4 ppt. While at
station II around 20-30 ppt with an average value of
21.1 ppt. In station I, there are many mangrove
forests that have been converted into fish ponds. The
management of fish ponds there are pumps that take
Accumulation of Heavy Metals of Cooper (Cu) and Lead (Pb) on Rhizophora mucronata in Mangrove Forest, Nelayan Village Sub Medan
Labuhan Subdistrict and Jaring Halus Village, Secanggang Subdistrict, No
243
sea water and put into ponds which affect salinity in
the area.
According to Hutagalung (1991) a decrease of
salinity and pH as well as an increase of temperature
caused a greater bioaccumulation rate because of the
increasing availability of metals.
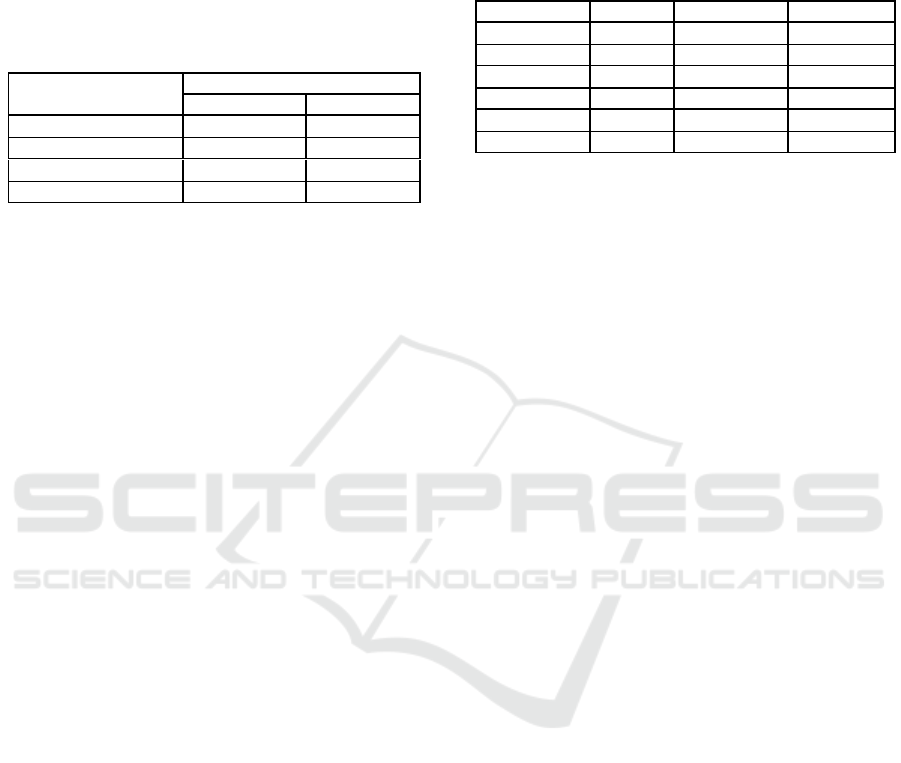
Table 1: Aquatic Environment Conditions Analyze.
Parameter
Station
I
II
pH Water
7
7.3
Salinity (ppt)
21,1
23.4
Water temp (°C)
27,3
28.1
Air temp (°C)
31.6
33.23
3.2 Heavy Metal Content of Cu and Pb
in the Roots, Leaves and Barks of
R. mucronata
Based on the results of measurements of heavy
metals Cu and Pb on the barks, roots and leaves of
the R.mucronata tree, showed that the barks, roots
and leaves were higher accumulating Cu than in Pb
metal (Table 2).
The results of measurements of heavy metals Cu
and Pb on the roots of R. mucronata trees showed
lower results compared to the content of the barks
and leaves. At station I the average content of Cu in
the roots of R. mucronata tree is around 5,033
mg/kg. The average content of Pb is around 0.884
mg/kg. At station II, content of Cu in the roots of R.
mucronata tree is around 2,740 mg/kg. The average
content of Pb is around 0.899 mg/kg. This is because
the roots do not store the substances that have been
absorbed from the soil for a long time. Though, it
translocated to the stem, leaves, and fruits (Priyanto
and Prayitno, 2004)
Based on the measurements of heavy metals Cu
and Pb on the leaves of R.mucronata tree, the results
were quite high. At station I, the average content of
Cu in the leaves of R. mucronata trees is 7.697
mg/kg. The content of Pb is 1,160 mg/kg. At station
II, the average metal content of Cu is 12,951 mg/kg.
The average content of Pb is 1.138 mg/kg. The
content of Cu at station II is higher than it is at
station I.
The heavy metal content of Cu on the bark of
station I is less than at station II. This is caused by
differences in tree diameter at both stations.
Diameter range at station I is 10.2 cm to 13.8 cm.
While the range of trees at station II is 17.5 cm to
22.6 cm. The difference in tree diameter determines
the amount of heavy metals and other substances
that accumulate in the tree. The bigger the diameter
of the tree, the bigger ability of the tree to
accumulate heavy metals and other substances.
Table 2: Average Analysis of Heavy Metal Content of Cu
and Pb in roots, leaves and barks of R.mucronata.
Sample
Station
Cu (mg/kg)
Pb (mg/kg)
Roots
I
5.033
0.884
Roots
II
2.740
0.899
Leafs
I
7.697
1.160
Leafs
II
12.951
1.138
Barks
I
8.357
1.115
Barks
II
21.734
2.480
3.3 Content of Heavy Metals Cu and
Pb in the Water and Sediment
The content of heavy metals Cu and Pb in water and
sediment in the water in the mangrove forest area of
Jaring Halus Village is higher than the ones in the
Nelayan Village Mangrove Forest. The average
content of Cu and Pb in sediments in the Mangrove
Forest Village of the Fishermen area is higher than
those in Jaring Halus Mangrove Forest (table 3).
By the results of measurements of Cu and Pb
heavy metals in water at both sampling stations, it
can be seen that the content of Cu heavy metal has a
higher concentration than the content of Pb metal.
This is due to the origin of Cu metal pollution which
is the main industrial waste that is above the
sampling location at station I, the waste area of
private oil palm plantations and community-owned
agriculture at station II. Sea transportation activities
contribute to Cu heavy metals in the environment
but in doses that are not too huge. At station II, there
is an oil palm management industry and sea
transportation activities to connect the Jaring Halus
Village at Secanggang District. In addition, Pb
heavy metals in the environment are generally
obtained from transportation activities and industrial
activities. At the station I, there are a few sea
transportation activities but there are numerous
industrial activities in the Medan Industrial Area
which is above the sampling location of station I.
From the results of measurement of Cu heavy
metals at station I, the average data obtained as
0.0439 L/kg. While at station II the average data was
obtained as 0.0496 L/kg. the content of Cu heavy
metal at station II is higher than station I. This can
be caused due to differences in sampling. At the
time of sampling at station I, it was carried out when
the dead tide (a small amount of tide entered the
location). At station II the sampling was carried out
during high tides (high tide entered at that location).
From the measurement content of Pb heavy metal at
ICONART 2019 - International Conference on Natural Resources and Technology
244
station I, the data average obtained as 0.0137 L/kg,
while in station II the average data obtained as
0.02457 L/kg. The content of Cu and Pb heavy
metals in both stations have exceeded the limit set
for seawater quality standards, 0.008 L / kg. (KLPM
KEPMEN No. 51 of 2004) .
From the results of measurements content of Cu
and Pb heavy metal in sediments at both sampling
stations obtained the content of Cu heavy metal with
average of 0,9003 mg/kg. While in station II, the
content of Cu heavy metal obtained with an average
of 0.776 mg/kg. the content of Cu heavy metal at
station I is higher than station II because at station I
there are many industrial activities and at station II it
is likened to control. Cu pollution at both stations
can still be tolerated (IADC/CEDA 1997).
From the measurement content of Pb heavy
metal at station I, the average data obtained as
2.7588 mg/kg, while in station II the average data
obtained as 0.9003 mg/kg and still included the
tolerance limit (IADC / CEDA 1997).
The content of heavy metal in sediments is
higher than it is in water. This can occur because of
sedimentation in sediments during heavy metal
content in high water. Heavy metals have properties
that easily bind organic matter and settle in the
bottom of the water and bind to sedimentary
particles. So that, the content of heavy metal in
sediments is higher it is in water.
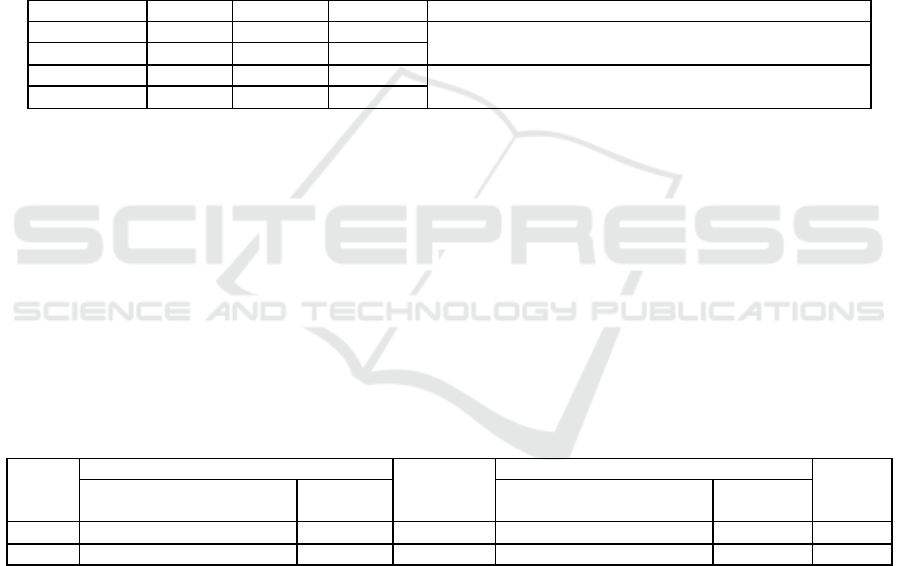
Table 3: Average Analysis content of Heavy Metal of Cu and Pb in Water and Sediments.
Sample
Station
Cu (mg/kg)
Pb (mg/kg)
Quality Standart
Water
I
0.0439
0.0137
KEPMEN KLH No. 51, 2004 (0,008 mg/l).
Water
II
0.0496
0.02457
Sediments
I
0.9003
2,75883
IADC/CEDA 1997, Cu (600 mg/kg) and Pb (1000 mg/kg).
Sediments
II
0.776
0.9003333
3.4 Bioconcentration Factor (BCF) To
Analyze the Ability of R. mucronata
in Accumulating Heavy Metal Cu
and Pb.
Based on the calculation of the value of bio
concentration factor (BCF), it is known that the
highest BCF value is for Cu metal which is 754,524
and the lowest BCF value is 188,527 for Pb metal.
The value of the Cu and Pb bio concentration factors
in two stations can be seen in Table 4.
From the results of the calculation of the bio
concentration factor values for heavy metals Cu at
the first station it can be concluded that the ability of
R. mucronata to accumulate Cu metal is better than
Pb metal. For station I Cu metal BCF values amount
to 480,357 and for Pb metals amount to 230,533. At
station II, the BCF value of Cu metal is 754,524 and
for Pb metal is 188,527. In accumulating Cu R.
mucronata metals are categorized as medium while
in accumulating Pb it is categorized as low.
Table 4: Value of Cu and Pb Bio concentration (BCF) Factors in Nelayan Village and Jaring Halus Village.
Station
Cu Consentration
Bcf Cu
(L/Kg)
Pb Consentration
Bcf Pb
(L/Kg)
Plant = Total Root, Bark
And Leaves (Mg/Kg)
Water
(L/Kg)
Plant = Total Root, Bark
And Leaves (Mg/Kg)
Water
(L/Kg)
I
21.0877
0.0439
480.357
3.1583
0.0137
230.533
Ii
37.4244
0.0496
754.524
4.6321
0.02457
188.527
4 CONCLUSION
The content of heavy metal Cu in R. mucronata
roots in Nelayan Village (5,033 mg/kg) are higher
than Jaring Halus Village (2,740 mg / kg), while for
the content of Pb in Jaring Halus Village (0,899
mg/kg) are higher than Nelayan Village (0.884
mg/kg). The content of Cu in R. mucronata leaves
are higher in jaring Halus Village (12,951 mg/kg)
than in Nelayan Village (7,697 mg/kg), while for the
content of Pb are higher in Nelayan Village (1,160
mg/kg) than in Jaring Halus Village (1,138 mg/kg).
The content of Cu in R. mucronata barks in Jaring
Halus Village (21.734 mg/kg) are higher than
Nelayan Village (8.35 mg/kg), Pb content in Jaring
Halus Village (2.480 mg/kg) are higher than in
Nelayan Village (1.115 mg/kg).
The ability of R. mucronata in accumulating Cu
heavy metals in Nelayan Village and Jaring Halus
Village is categorized as medium with BCF values
of 480.357 and 754.524, whereas in accumulating
Pb heavy metals in Nelayan Village and Jaring
Accumulation of Heavy Metals of Cooper (Cu) and Lead (Pb) on Rhizophora mucronata in Mangrove Forest, Nelayan Village Sub Medan
Labuhan Subdistrict and Jaring Halus Village, Secanggang Subdistrict, No
245

Halus Village is categorized as low with BCF values
of 230,533 and 188,527.
ACKNOWLEDGEMENTS
Universitas Sumatera Utara esearch Institute In
accordance with the Universitas Sumatera Utara
TALENTA Research Implementation Contract
Number: 2590 / UN5.1.R / PPM / 2018, March 16,
2018.
REFERENCES
Amin, B. 2001. Accumulation and Distribution of Pb and
Cu Heavy Metals in Mangroves. (Avicennia marina)
on the waters of Dumai Beach, Riau. UNRI Press.
Riau.
Arisandy, K. R., Herawati, E. Y., Suprayitno, E. 2012.
Accumulation of Lead Heavy Metal (Pb) and
Histology Picture on Avicennia marina (forsk.) Vierh
Network in East Java Coast Waters. Journal of
Fisheries Research 2012.
Bengen, D. G. 2000. Introduction and management of
mangrove ecosystems. Center for IPB Coastal and
Ocean Resources Studies. 58 p.
West Java BPLHD. 2013. Lead Pollution.
http://www.bplhdjabar.go.id/index.php/bidang
pengendalian/subid-pemonitor-pencemaran/168-
pencemaran-pb-timbal. [January 17 2013].
Dahlan, E. N. 1986. Tea Leaf Pollution by Lead as a
Result of Motor Vehicle Emissions at Gunung Mas
Puncak. Papers of the Indonesian Science Congress,
MAB National Committee, Jakarta.
Dahlan, E. N. 1989. Study of Plant Ability to Absorb and
Absorb Lead Emissions from Motorized Vehicles.
Thesis. Postgraduate School, Bogor Agricultural
University. 102 p
Dahlan, Z., Sarno, A. Barokah. 2009. Architectural Model
of Lateral Roots and Mangrove Roots (Rhizophora
apiculata Blume). Journal of Science Research 12
(2).
Duke, N. C. 2006. Rhizophora apiculata, R. mucronata, R.
Stylosa, R. x annamalai, R. x lamarckii (Indo-West
Pacific stilt mangrove). Permanent Agriculture
Resources 2 (1).
Hamzah, F., Setiawan, A. 2010. Accumulation of Pb, Cu
and Zn Heavy Metals in Muara Angke Mangrove
Forest, North Jakarta. Journal of Tropical Marine
Science and Technology. 2: 41-52.
Handayani, T. 2006. Bioaccumulation of heavy metals in
Rhizophora mucronata and Avisennia marina
mangroves in Muara Angke Jakarta. Center for
Environmental Technology Agency for the
Assessment and Application of Technology.
Hoshika, A., Shiozawa, T., Kawana, K., Tanimoto, T.,
1991. Heavy Metal Pollution in Sediment from the
Seto Island, Sea, Japan. Marine Pollution Bulletin 23:
101-105.
Hutagalung. H. P. 1991. Marine Pollution by Heavy
Metals. Research and Development Center for
Oceanology. Status of Marine Pollution in Indonesia
and Monitoring Techniques. LIPI. Jakarta.
Harty, C. 1997. Mangroves in New South Wales and
Victoria. Vista Publications, Melbourne, 47pp.
Irwanto, 2008. Benefits of Mangrove Forests.
www.irwantoshut.co.cc. [July 12, 2012]
Karimah, A., Gani, A. A, Asnawati. 2002. Profile of Lead
(Pb) Heavy Metal Content in Kupang Rice Shells
(Tellina versicolor). MIPA Faculty of University of
Jember. East Java.
Kusmana, C. 2010. Mangrove Response to Pollution. IPB
Press. Bogor.
MacFarlane, G. R., Pulkownik, A., Burchett, M. D. 2003.
Accumulation and Distribution of Heavy Metals in
gray mangrove, Avicennia marina (Forsk.) Vierh:
Biological indication potential. Environmental
pollution, Vol. 123, pp. 139-151.
Mason, C. F. 1981. Biology of fresh water pollution.
Longman. New York. 351p.
Merian, E. 1994. Toxic Metal In The Environment. VCH
Verlagsgeselischatt mbH. Weinheim.
Moore J. W, Ramamoorthy S. 1984. Heavy metal in
natural waters. Springer-velag. New York, Berlin,
Heidelberg. Tokyo 268p.
Nybakken, J. W. 1992. Marine biology. An ecological
approach. Gramedia, Jakarta. Translator: Eidman et
al.
Panjaitan, G. S., Dalimunthe A., Yunasfi. 2008.
Accumulation of Heavy Metal Copper (Cu) and Lead
(Pb) in Avicennia marina trees in Mangrove Forests.
Thesis. University of North Sumatra. Medan.
Perales-Vela H. V., Gonzalez M. S., Montes H.,
Canizares, V. R. O. 2007. Growth, photosynthetic
and respiratory responses to sub-lethal copper
concentrations in Scenedesmus incrassatulus
(chlorophyceae). Chemosphere. 67: 2274-2281.
Priyanto B, Prayitno J. 2006. Phytoremediation as a
Pollution Recovery Technology, Especially Heavy
Metal. http://ltl.bppt.tripod.com/sublab/lflora1.htm.
[October 1, 2013]
Riani, E., 2004. Utilization of Green Shellfish as Biofilter
for the Waters of Jakarta Bay. DKI Jakarta Regional
Government.
Riani, E. 2011a. The Role of Women in Environmental
Conservation Efforts. Technical Guidance for
Women's Empowerment Programs for Women's
Organizations. Director General of Community and
Village Empowerment, Ministry of Home Affairs,
October 19, 2011.
Riani, E. 2011b. The Role of Education in Building
Environmentally Friendly Behaviors (Green
Behavior) for Realizing Government Promises
Reduces GHG Emissions by 26%. Nizam and Munir
E (Editors). XXI Century Ethics: The Role of
Humans in Climate Change, DPT-Director General
ICONART 2019 - International Conference on Natural Resources and Technology
246

of Higher Education. Ministry of Education and
Culture (in-press).
Riani, E. 2011c. Impact of Climate Change on
Reproduction, Safety and Food Security. Nizam and
Munir E (Editors). XXI Century Ethics: The Role of
Humans in Climate Change, DPT-Director General
of Higher Education. Ministry of Education and
Culture (in-press).
Riani, E. 2012. Climate Change and Aquatic Biota. IPB
Press. Bogor.
Santoso, N. 2000. Pattern of Mangrove Ecosystem
Monitoring, Paper Presented at the National
Workshop on the Development of the 2000 Marine
Ecosystem Monitoring System. Jakarta, Indonesia.
Santoso. N. H. W. Arifin. 2004. Rehabilitation of
Mangrove Forests on Green Lanes in Indonesia.
Mangrove Assessment and Development Institute
(LPP Mangrove). Jakarta, Indonesia.
Soemirat, J. 2003. Environmental toxicology. Gajah Mada
University Press. Yogyakarta.
Tomlinson, P.B. 1986. The botany of mangrove.
Cambridge University Press. Cambridge.
Vogel. 1994. Qualitative Inorganic analysis. Department
of Chemistry Queens University. Belfast, N. Ireland.
Walsh, G.E. 1974. Mangroves: A review. In Reinhold, R.
J. and W.H. Queen (ed.). Ecology of Halophytes.
New York: Academic Press.
Wisnubroto, S, S.S.L Aminah, and Nitisapto, M. 1982.
Principles of Agricultural Meteorology, Department
of Soil Science, UGM. Yogyakarta, and Ghalia
Indonesia. Jakarta.
Yruela. 2005. Cooper in Plannts. Braz. J. Hydrol.
144: 405-42.
Yudhanegara, R. A. 2005. Absorption of Pb and Hg
Heavy Metal Elements by Hyacinth [Eichhornia
crassipes (Mart). Solms] and Kiapu (Pistia stratiotes
Linn). Thesis. Department of Forest Resources
Conservation IP
B. Bogor.
Accumulation of Heavy Metals of Cooper (Cu) and Lead (Pb) on Rhizophora mucronata in Mangrove Forest, Nelayan Village Sub Medan
Labuhan Subdistrict and Jaring Halus Village, Secanggang Subdistrict, No
247
