
Protein Induced as Salinity Stress in Elaeis guineensis Jacq.
Dolly Sojuangan Siregar
1
, Mohammad Basyuni
2
and Rahmah Hayati
3
1
Department of Agrotechnology, Faculty of Agriculture, Universitas Samudra, Meurandeh, Langsa City, Aceh, Indonesia
2
Department of Forestry, Faculty of Forestry, Universitas Sumatera Utara, Jl. Tri Dharma Ujung No. 1 Medan, North Sumatera
20155, Indonesia
3
Graduate School of Agrotechnology, Faculty of Agriculture, Universitas Sumatera Utara, Jl. Dr. A Sofyan No. 3 Medan, North
Sumatera 20155, Indonesia
Keywords: Oil Palm, Plasma Membrane, Salinity.
Abstract: Palm oil plants (Elaeis guineensis) are oil-producing plants grown in the tropics. Palm oil is sensitive to low
temperatures but high tolerance to salinity stress and drought. The present work evaluates of the bioinformatics on
the NCBI database as well as expected the physicochemical of protein salinity. There is three protein salinity induced
from E. guineensis deposited in NCBI. Length of genes was between 525 to 633 bp. The same Molecular weight at
X1 and X2 was 5945.24, but it is different from X3 which was 18052.17. Chloroplast transit peptide ranged from
0.142 and 0.445. Reactive oxygen species (ROS) plays a crucial role in promoting mitochondrial peptide targets in
plants induced by salinity, ranging from 0.022 to 0.110. These results d variations and roles of different physical and
chemical characteristics of amino acids in protein due to salinity stress in oil palm plants.
1 INTRODUCTION
Salinity is a major problem that affects the world's
agricultural output and ecosystems. In latest decades,
elevated soil salinity has transformed global farming
barriers (Rengasamy, 2006; Munns and Tester, 2008).
About 50% of the world's land will become salt by the
21st century (Mahajan and Tuteja 2005).
One of the most notable concerns of osmotic stress
in plants is the production of high quantities of reactive
oxygen species (ROS), followed by oxidative damage,
e.g. protein, lipid, pigment, and DNA degradation. (Das
and Roychoudhury 2014).
Palm oil (Elaeis guineensis) is susceptible to low
temperatures but has a high tolerance to salt stress and
drought (Cao et al., 2011). By identifying and validating
genes associated with salinity stress responses in oil
palm, it will help through molecular breeding.
The first sensory mechanism that senses salt
stimulation is two elements that are seen during salinity
stress circumstances, namely hyperosmotic stress and
Na+ ion toxicity. As a result of salinity stress, plants
produce Ca
2
+ and ROS which are secondary
messengers. The main organ of the plant that feels the
salinity stress is the root. Plasma membranes and
cytoplasmic proteins, G proteins, Ca2 + binding
proteins, phosphoproteins and ethylene receptors
intermediate the process. (Ghosh and Xu 2014).
At present, it is indispensable to recognize the nature
of the mechanism of salt adaptation in oil palm plants to
advance future oil palm varieties that are tolerant of
salinity. The discovery of salt-tolerant genes will help
breeders select parent species (germplasm) and
progenies using marker aid. The results of the
physiological and multi-OMICS analysis may deliver
responses to the subsequent major questions:
1. Are some existing oil palm varieties adaptive to salt
stress?
2. What is the mechanism for the adaptation of salt
stress to oil palm plants?
3. Are there differences between sporogenous tissue
in roots and leaves during salt stress?
4. Does the oil palm salinity gene affect the
phenotype?
5. What is related to the modification of
posttranslational salinity tolerance in oil palm
plants?
The present study, therefore, evaluates of the
bioinformatics on the NCBI database as well as
expected the physicochemical of protein salinity from
E. guineensis.
112
Siregar, D., Basyuni, M. and Hayati, R.
Protein Induced as Salinity Stress in Elaeis guineensis Jacq..
DOI: 10.5220/0008526301120115
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 112-115
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIAL AND METHOD
2.1 Materials
Three salinity genes on oil palm acquired from NCBI.
The DNA and amino acid references used in the
research follow:
1. XM_019850660.1 GI: 1130661850 Salt stress-
induced hydrophobic peptide ESI3-like [E.
guineensis]; NCBI Reference Sequence:
XP_019706219.1
2. XM_019846899.1 GI: 1130626415 Salt stress-
induced hydrophobic peptide ESI3-like [E.
guineensis]; NCBI Reference Sequence:
XP_019702458.1
3. JZ142439.1 GI: 527498159 EgFLSTP6 E.
guineensis cDNA similar to salt tolerance protein
6, mRNA sequence 525 bp linear mRNA
2.2 Physicochemical Features of the
Salinity Protein of Oil Palm
Online Protparam (web.expasy.org/protparam/) has
been used to control the composition,
physicochemical features of oil palm plants ' protein
salinity. The calculated factors are the length of
genes/bp, molecular weight, theoretical isoelectric
values points, a total number of atoms, total
negatively charged residues, total number of
positively charged residues, instability coefficient,
aliphatic index, and grand mean of hydropathicity as
prior described (Basyuni et al., 2017)
2.3 Peptide Transfer and Subcellular
Localization of Protein-induced
Salinity Proteins in Oil Palm Plants
Peptide predictions transit through the online P1.1
target server (www.cbs.dtu.dk/services/targetp/).
Peptide chloroplast transit, mitochondrial target
peptide, the signal peptide of the secretory pathway,
reliability indicator were found. Online PSORT
(predictive tool for subcellular localization of
proteins) (psort.hgc.jp/form.html) is used to analyze
the subcellular of protein dehydration which is
induced by salinity stress in oil palm plants as earlier
shown (Basyuni and Wati, 2017).
2.4 Phylogenetic Analysis of Protein-
induced Salinity in Oil Palm
Locus numbers of the sequence of the physical and
chemical characteristic of oil palm protein induced
salinity used this investigation in this manner:
E.guineensis XM_019850660, XM_019846899, and
JZ142439.1. Phylogenetic analysis of amino acid
arrangement from of protein-induced salinity in oil
palm was carried out with CLUSTAL W ver. 1.83
(Thompson et al., 1994) of the DNA Data Bank of
Japan (Mishima, Shizuoka, Japan) accompanied by
depicting with TreeView, ver. 1.6.6 (Page, 1996)
according to a neighbor-joining method. Bootstrap
analysis with 1000 repetitions was used to weigh the
strong point of the knots in the tree (Felsenstein.
1995).
3 RESULT
3.1. Physicochemical Characteristics of
the Protein Induced Salinity in E.
guineensis
The physicochemical activities of a protein are
analyzed by the similar properties of the amino acids
in it. Each protein molecule contains of a long chain
of amino acid residues and is connected by peptide
bonds. Table 1 showed the several parameters of
physicochemical protein induced salinity in
E.guineensis. Length of genes between 525 to 633 bp.
The same Molecular weight at X1 and X2 is 5945.24,
but it is different from X3 which is 18052.17.
Theoretical isoelectric points values at X1 and X2 are
almost close to 4.65 and 4.0 but different from X3
which is 8.69. The total number of atoms at X1 and
X2 is almost close to 8.64 and 8.71 three times
different from X3 which is 2478.
The total number of negatively charged residues
X1 and X2 which are 3 and 4 are different from X3
which is 11. The total number of positively charged
residues on X1 and X2 is the same, namely 1, but
different from X3, namely 14, Instability coefficient
at X1, X2 and X3 between 27.15 and 31.66. Aliphatic
indexes at X1 and X2 are 162.42 and 165.93 but are
low at X3 which is 60.18. The hydrophobicity is a
significant stabilization force in protein folding.
Grand average of hydropathicity on X1 and X2 is
1.604 and 1.491 while X3 is -0.443. Despite the
availability of thousands of stress associated ESTs of
in E. guineensis (Low et al., 2008), quantitative gene
expression analysis of these genes is only recently
attempted for the identification of candidate
genes/factors that are contributing to salinity
tolerance. With the advent of the qPCR technique, it
is easier to quantify each gene and establish its
relevance under the given stress situations.
Protein Induced as Salinity Stress in Elaeis guineensis Jacq.
113
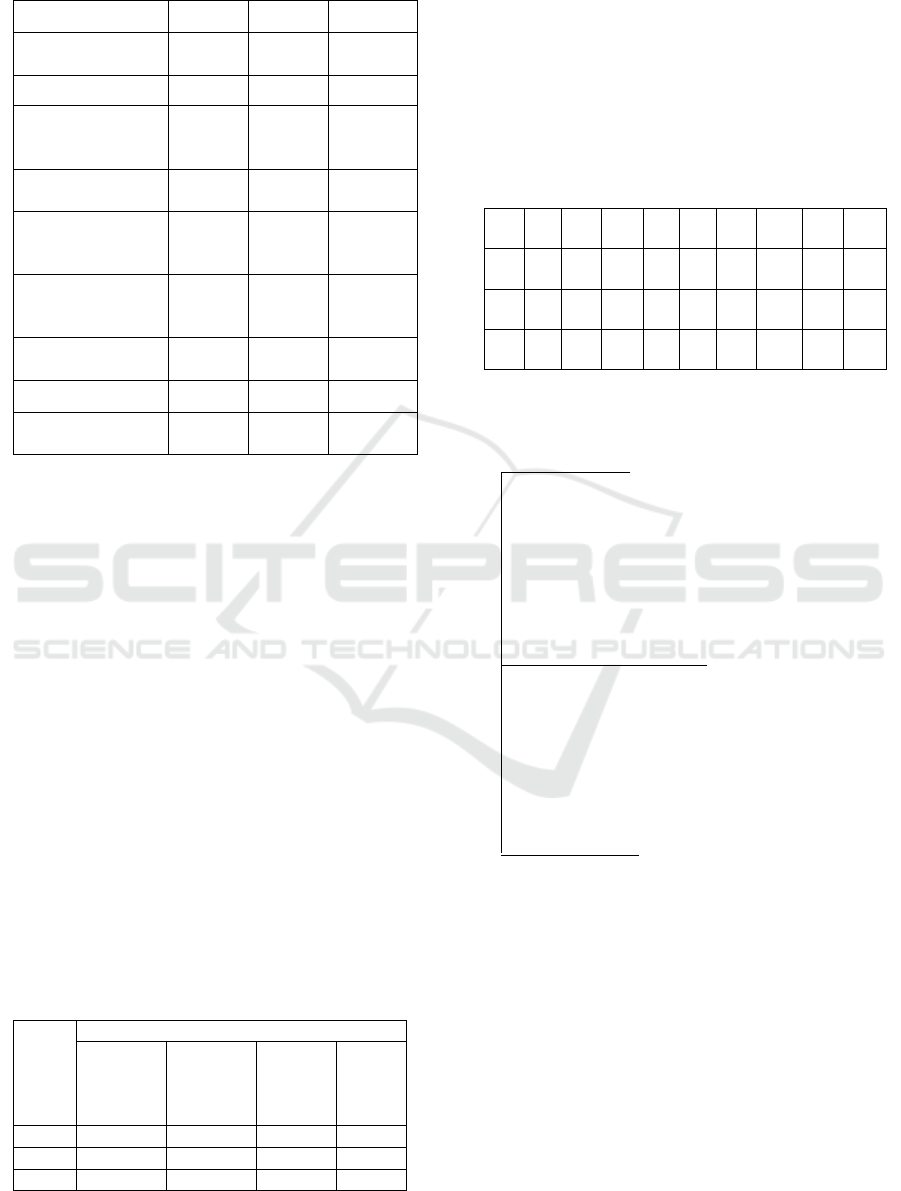
Table 1: Physicochemical characteristic of the protein
induced salinity in E.guineensis.
Variant
X1
X2
X3
Length of
genes/bp
630
633
525
Molecular weight
5945.24
5945.24
18052.17
Theoretical
isoelectric points
values
4.65
4.00
8.69
Total number of
atoms
864
871
2478
Total number of
negatively
charged residues
3
4
11
Total number of
positively charged
residues
1
1
14
Instability
coefficient
27.15
31.66
27.54
Aliphatic index
162.41
165.93
60.18
Grand average of
hydropathicity
1.604
1.491
-0.443
3.2. Potential Transfer of Peptide and
Subcellular Location
The potential for a prospective transfer peptide in E.
guineensis is shown in Table 2. Four reliability
factors were identified: chloroplast transit peptide,
mitochondrial target peptide, secretory path signal
peptide, and prediction of reliability. Chloroplast
transit peptide ranges from 0.142 and 0.445. ROS
plays a crucial role in promoting mitochondrial
peptide targets in plants that are gripped by salinity
(Huang et al., 2016), ranging from 0.022 to 0.110.
The signal peptide of the secretory pathway can be
found in terminal N and terminal C of protein and
most cases stored in mature proteins with values
ranging from 0.154 and 0.670. Reliability of X1, X2
and X3 predictions ranges between 3 and 5. These
indicated that ROS-scavenging enzymes played
crucial roles in salinity tolerance mechanism (Joseph
et al., 2011).
Table 2: The promising of potential transit peptide induced
salinity in E. guineensis.
Varian
t
Reliability
Chloroplast
transit peptide
Mitochondrial
target peptide
Signal
peptide of
secretory
pathway
Reliabilit
y
predictio
n
X1
0.142
0.022
0.670
3
X2
0.445
0.076
0.154
4
X3
0.291
0.110
0.170
5
Table 3 shows the subcellular location of protein-
induced salinity in E. guineensis. There are two (2)
variants (X1, X2) were located in the vacuole (Vac),
Plasma (Plas), and extracellular (Extr). One variant
(X1) in Endoplasmic Reticulum (ER), Golgi (Golg).
One variant (X3) were located in Mitochondrial (Mito),
Cytoplasm (Cyto), Nucleolus (Nucl) and Chloroplast
(Chlo).
Table 3: Subcellular localization of protein-induced salinity
in E.guineensis.
Va
r
Vac
ER
Pla
s
Golg
Extr
Mito
Cyt
o
Nu
cl
Chl
o
X
1
9
2
1
1
1
N
d
nd
nd
nd
X
2
1
1
N
d
1
n
d
2
N
d
nd
nd
nd
X
3
n
d
N
d
nd
n
d
n
d
1
2
2
9
Note: Vac: Vacuole, ER: Endoplasmic Reticulum, Plas: Plasma
Golg: Golgi, Extr: Extracellular, Mito: Mitochondrial, Cyto
Cytoplasm, Nucl: Nucleolus, Chlo: Chloroplast, nd: not
detected
Figure 1: Phylogenetic tree among observed genes.
Figure 1 illustrates the phylogenetic among the
gene observed. There are three branches
representative by each gene. To further understand
the genes, several additional genes related to salinity
from E. guineensis are needed.
4 CONCLUSIONS
These findings stated differences and roles in salt-
induced amino acids in oil palm of various physical
XP 019706219.1
XP 019702458.1
JZ142439.1
0.1
ICONART 2019 - International Conference on Natural Resources and Technology
114

and chemical features. The promising prospective
transit peptide, subcellular location of protein genes
owing to the stress of salinity in E. guineensis.
ACKNOWLEDGEMENTS
This research was partially endorsed by a Penelitian
Strategis Nasional Institusi (PSN Institusi 2019)
(PSN Institusi 2019) from the Directorate for
Research and Community Service, Ministry of
Research, Technology and Higher Education,
Republic of Indonesia.
REFERENCES
Basyuni, M., Wasilah, M., and Sumardi. 2017.
Bioinformatics study of the mangrove actin genes
Journal of Physics Conference Series 801, 012013
Basyuni, M. and Wati, R., 2017. Bioinformatics analysis of
the oxidosqualene cyclase gene and the amino acid
sequence in mangrove plants. In Journal of Physics:
Conference Series 801, 012011.
Cao, H. X., Sun, C. X., Shao, H. B., and Lei, X. T. 2011.
Effects of low temperature and drought on the
physiological and growth changes in oil palm seedlings.
African Journal of Biotechnology, 10(14), 2630-2637.
Das K, and Roychoudhury A. 2014. Reactive oxygen
species (ROS) and response of antioxidants as ROS-
scavengers during environmental stress in plants.
Frontiers in Environmental Science 2:53.
Felsenstein, J., 1985. Confidence limits on phylogenies: an
approach using the bootstrap. Evolution, 39(4), pp.783-
791.
Ghosh, D. and Xu, J., 2014. Abiotic stress responses in
plant roots: a proteomics perspective. Frontiers in Plant
Science 5, 6.
Huang, S., Van Aken, O., Schwarzländer, M., Belt, K. and
Millar, A.H., 2016. The roles of mitochondrial reactive
oxygen species in cellular signaling and stress response
in plants. Plant physiology, 171(3), 1551-1559.
Joseph, B., Jini, D. and Sujatha, S., 2011. Development of
salt stress-tolerant plants by gene manipulation of
antioxidant enzymes. Asian Journal of Agricultural
Research, 5(1), 17-27.
Low, E.T.L., Alias, H., Boon, S.H., Shariff, E.M., Tan,
C.Y.A., Ooi, L.C., Cheah, S.C., Raha, A.R., Wan, K.L.
and Singh, R., 2008. Oil palm (Elaeis guineensis Jacq.)
tissue culture ESTs: identifying genes associated with
callogenesis and embryogenesis. BMC Plant Biology,
8(1), p.62.
Mahajan S, and Tuteja N. 2005. Cold, salinity and drought
stress: an overview. Archives of Biochemistry and
Biophysics 444,139-158.
Munns, R. and Tester, M., 2008. Mechanisms of salinity
tolerance. Annual Review of Plant Biology 59, 651-681.
Page, R.D., 1996. Tree View: An application to display
phylogenetic trees on personal computers.
Bioinformatics, 12(4), pp.357-358.
Rengasamy, P., 2006. World salinization with emphasis on
Australia. Journal of Experimental Botany, 57(5),
1017-1023.
Thompson, J.D., Higgins, D.G. and Gibson, T.J., 1994.
CLUSTAL W: improving the sensitivity of progressive
multiple sequence alignment through sequence
weighting, position-specific gap penalties and weight
matrix choice. Nucleic acids research, 22(22), pp.4673-
4680.
Protein Induced as Salinity Stress in Elaeis guineensis Jacq.
115
