
Influence of Ions and Temperatures on Water-Ethanol-Gasoline
Liquid-Liquid Equilibrium
Okta Bani
1
, Lilis Sukeksi
1,2
, Taslim
1
, Iriany
1,2
and Muhammad Dedi Anggreawan
1
1
Department of Chemical Engineering, Universitas Sumatera Utara, Jl Almamater, Medan, Indonesia
2
Center of Excellence for Natural Resources Based Technology, Universitas Sumatera Utara, Medan, Indonesia
mhddedianggreawan@yahoo.com
Keywords: Water-Ethanol-Gasoline Equilibrium, Ions, KH
2
PO
4
, KCl, KOH.
Abstract: Water-ethanol-gasoline liquid-liquid equilibrium (LLE) was investigated. In this study, the LLE data was
obtained gravimetrically and volumetrically by turbidity titration under influence of KH
2
PO
4
, KCl, and
KOH. Effect of temperature was also evaluated at 30 and 50℃. Results suggest that presence of ions alter
the LLE and different ions affect the LLE differently. At concentration of 20 mM, temperature had
significant effect on the LLE. Increasing temperature reduced the heterogeneous region and allowed more
water in gasoline phase.
1 INTRODUCTION
World energy demand is predicted to increase with
Asian countries leading the trend (U.S. EIA, 2016).
To meet the energy demand, alternative energy is
currently being developed with lignocellulosic
ethanol as one of the potential candidate. Cellulosic
ethanol has gained popularity these recent years, but
it is yet to be commercialized because it is too
expensive and inefficient (Johnson, 2016; Olofsson
et al., 2017; Klein-Marcuschamer et al., 2011).
While many researches had been conducted
emphasizing on different aspects of the production
such as pretreatments (type and condition),
hydrolysis (enzyme type and load), fermentation
(microbes and condition), process scheme (SSF,
SScF, CBP, and DMC), pre fermentation processing
(hydrolysate detoxification, sugar pre concentration,
additives), cell/enzyme immobilization, and so on
(Li et al., 2017; Singh et al., 2017; Vohra et al.,
2014; Hernawan et al., 2017; Xu and Wang, 2017;
Kumar et al., 2016; Lam et al., 2014; Johnson, 2016;
Ziolkowska, 2014), few focused on alternative
purification step or its integration into the process
scheme. One possible scheme is production of
alcohol gasoline blend, known as gasohol, by
extraction of fermentation broth (Leeper and
Wankat, 1982; Lee and Pahl, 1985). Coupled with
direct broth recycling using non-sterile fermentation
(Chen and Wan, 2017), this method can reduce
operation cost to overcome the economic barrier of
cellulosic ethanol. Previous attempt on integration of
purification step and direct broth recycling using
biodiesel had shed some light on its viability, though
extraction using gasoline might be more
economically feasible (Bani, 2014). However,
integrating extraction using gasoline into process
scheme requires careful consideration due to
possible enzyme and microbe inhibition, formation
of emulsion, water infiltration into gasohol, etc. The
interdependency of these factors also further
complicates the analysis of such system. Therefore,
this study aimed to collect water-ethanol-gasoline
equilibrium data as foundation for the system
analysis.
2 MATERIALS & METHODS
2.1 Materials Collection
Aquabidest, ethanol, KOH, KCl, and KH2PO4 were
purchased from local chemical store (CV. Rudang
Jaya) while gasoline was purchased from local petrol
station. All chemicals used were of technical grade
and gasoline was Pertamax from PT. Pertamina.
Specification of Pertamax gasoline is tabulated in
Table 1.
100
Bani, O., Sukeksi, L., Taslim, ., Iriany, . and Anggreawan, M.
Influence of Ions and Temperatures on Water-Ethanol-Gasoline Liquid-Liquid Equilibrium.
DOI: 10.5220/0008525601000103
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 100-103
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
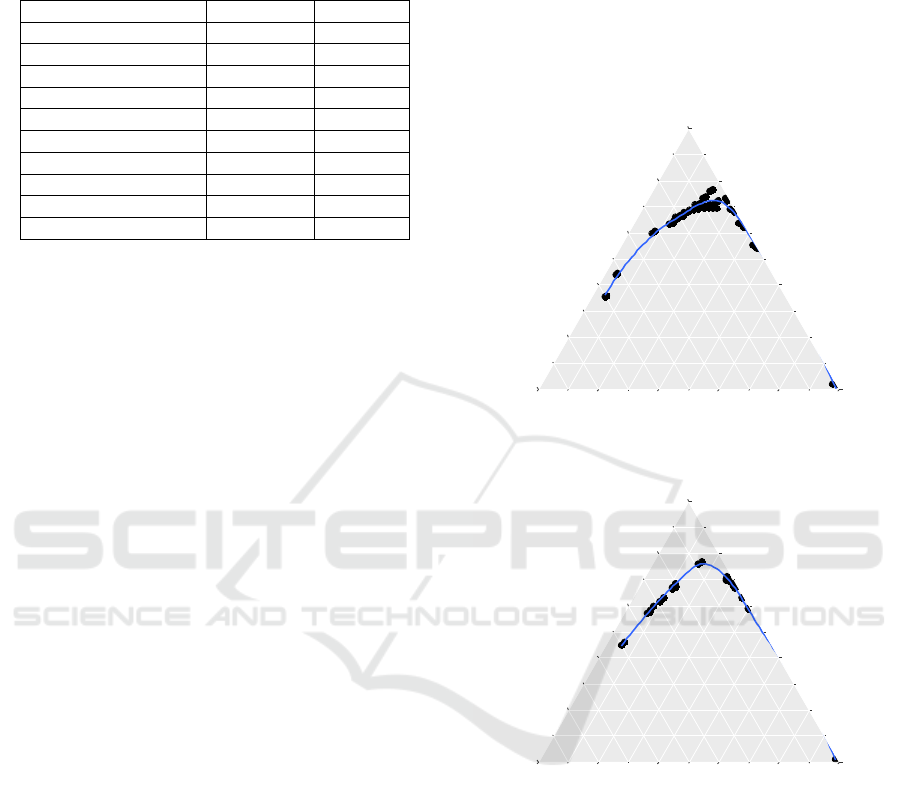
Table 1: Some Specification of Pertamax Gasoline
(Pertamina 2017).
Characteristics
Unit
Value
Octane number
RON
>92
Oxidation stability
min
>480
Sulfur content
% m/m
0.05
Lead content
g/L
0.013
Oxygen content
% m/m
<2.7
Aromatic content
% v/v
<50
Benzene content
% v/v
<5
Sediment
mg/L
<1
Colour
Blue
Die content
g/100 L
<0.13
2.2 Liquid Liquid Equilibrium Data
Collection
Water-ethanol-gasoline equilibrium data was
obtained by turbidity titration. The procedure began
by preparing a water-ethanol mixture in a 100-ml
flask. The mixture was titrated with gasoline using a
5-ml burette until it became turbid, then added again
with gasoline. Afterwards, it was titrated with
ethanol to clarity, then added again with ethanol.
Each phase change and liquid addition was recorded.
The titration was repeated until the flask was full.
Initial mixture composition was adjusted with each
procedure iteration to obtain enough data for
construction of ternary phase diagram. The
experiment was repeated under influence of
potassium ions (KOH, KCl, and KH
2
PO
4
) and
temperature of 30 and 50°C. Ion influence was
investigated by replacing aquabidest with ionic
solution at concentration of 20 mM. Temperature
variation was maintained within ±2°C by immersing
the flask in water bath during titration.
2.3 Ternary Phase Diagram
Construction
LLE Data was plotted in R using ggtern library
using gg_smooth_tern plotting (Hamilton, 2017).
LLE composition was calculated as mass fraction by
adding up each component during each phase
change. For gravimetric data, the mass change
during each addition of liquid was treated as added
mass of said liquid. For volumetric data, the titer
volume was converted to mass using the liquid
density at the room temperature during experiment.
3 RESULTS AND DISCUSSIONS
3.1 Effect of Potassium Ions
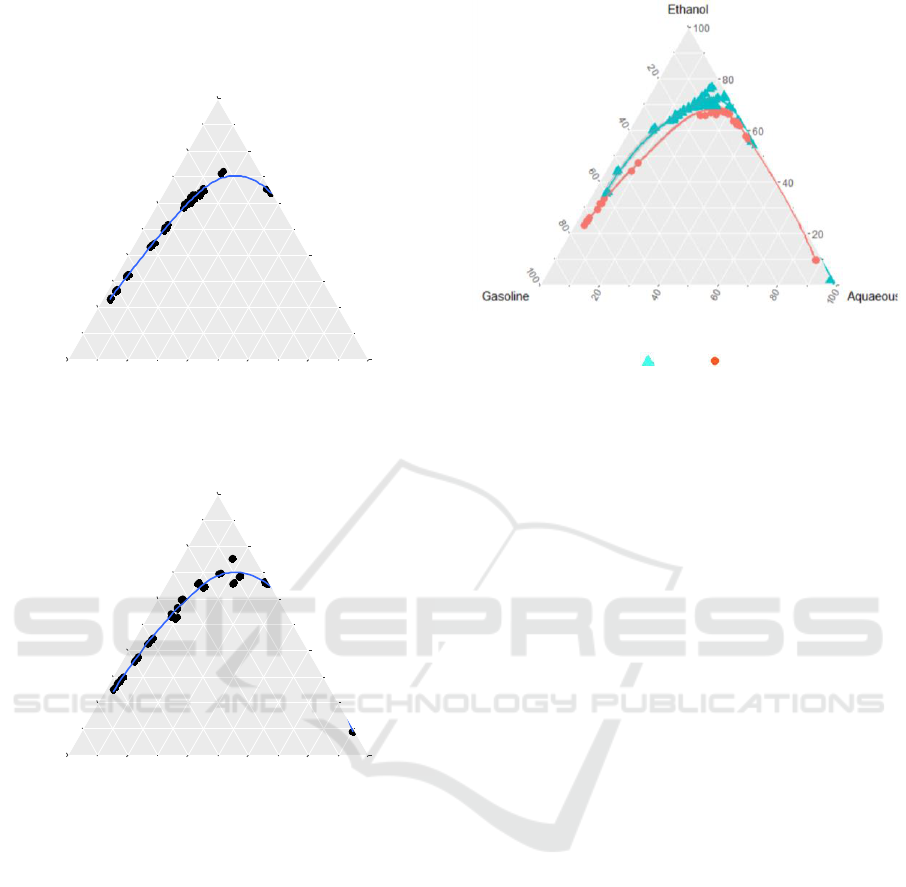
Ternary phase diagrams for water-ethanol-gasoline
system without presence of ions and under influence
of KOH, KCl, and KH
2
PO
4
are shown in Figure 1-4.
Figure 1: Water-Ethanol-Gasoline LLE System.
Figure 2: Effect of KOH on Water-Ethanol-Gasoline LLE
System.
Addition of KH
2
PO
4
increased the heterogeneous
region of the diagram, indicating that the system had
more separation. However, it is worth noting that
KH
2
PO
4
was emulsified in this system and as such,
require a more thorough investigation to reach better
conclusion. For KOH, the shape of heterogeneous
region became rounder. This resulted in less water in
the gasoline phase at low ethanol concentration
region but more water in gasoline phase at high
ethanol concentration region, which means that
KOH promote both separation and homogeneity. A
more sophisticated method is required to ascertain
this result. For KCl, its addition seemed to promote
20
40
60
80
100
20
40
60
80
100
20
40
60
80
100
Ethanol
Gasoline Aquaeous
Water
20
40
60
80
100
20
40
60
80
100
20
40
60
80
100
Ethanol
Gasoline Aquaeous
KOH
Influence of Ions and Temperatures on Water-Ethanol-Gasoline Liquid-Liquid Equilibrium
101
homogeneity as evidenced from the shrinking
heterogeneous region.
Figure 3: Effect of KCl on Water-Ethanol-Gasoline LLE
System.
Figure 4: Effect of KH
2
PO
4
on Water-Ethanol-Gasoline
LLE System.
3.2 Effect of Temperature
Ternary phase diagrams for water-ethanol-gasoline
system at 30°C and at 50°C are shown in Figure 5. It
is apparent that temperature has significant effects
on the LLE as the heterogeneous region shrank
significantly, which suggests that more water will
present in gasoline and more gasoline will present in
water. Similar finding at different temperature range
was also reported (Cerempei, 2011)
Figure 5: Effect of Temperature on Water-Ethanol-
Gasoline LLE System. : 30℃, : 50℃
4 CONCLUSIONS
Water-ethanol-gasoline LLE is influenced by
presence of ions and temperature. The most
pronounced of these two is temperature. Different
ions bode different effects on the system, although
more data are required to ascertain these results.
ACKNOWLEDGEMENTS
The authors would like to thank the Ministry of
Higher Education of Indonesia for the funding
through TALENTA USU 2017.
REFERENCES
Bani, O. 2014. Bioetanol production from enceng gondok
using hydrolisis process, fermentation and extraction.
Master. Universitas Sumatera Utara.
Cerempei, V. 2011. Temperature influence on phase
stability of ethanol-gasoline mixtures. Chem. J. Mold.,
6(1), pp. 69-72.
Chen, Z., Wan, C. 2017. Non-sterile fermentations for the
economical biochemical conversion of renewable
feedstocks. Biotechnology Letters, 39(12), pp.1–13.
Hamilton, N. 2017. An extension to “ggplot2”, for the
creation of ternary diagrams. Available at:
https://cran.r-project.org/package=ggtern [Accessed
November 30, 2017].
Hernawan, Maryana, R., Pratiwi, D., Wahono, S. K.,
Darsih, C., Hayati, S. N., Poeloengasih, C. D., Nisa,
K., Indrianingsih, A. W., Prasetyo, D. J., Jatmiko, T.
H., Kismurtono, M., Rosyidaet, V. T. 2017.
Bioethanol production from sugarcane bagasse by
simultaneous sacarification and fermentation using
20
40
60
80
100
20
40
60
80
100
20
40
60
80
100
Ethanol
Gasoline Aquaeous
KCl
20
40
60
80
100
20
40
60
80
100
20
40
60
80
100
Ethanol
Gasoline Aquaeous
KH2PO4
ICONART 2019 - International Conference on Natural Resources and Technology
102

Saccharomyces cerevisiae. In: AIP Conference
Proceedings. In p. 20026. Available at:
http://aip.scitation.org/doi/abs/10.1063/1.4978099
[Accessed March 30, 2017].
Johnson, E. 2016. Integrated enzyme production lowers
the cost of cellulosic ethanol. Biofuels, Bioproducts
and Biorefining, 10(2), pp.164–174. Available at:
http://doi.wiley.com/10.1002/bbb.1634 [Accessed
March 30, 2017].
Klein-Marcuschamer, D., Simmons, B. A., Blanch, H.W.
2011. Techno-economic analysis of a lignocellulosic
ethanol biorefinery with ionic liquid pre-treatment.
Biofuels, Bioproducts and Biorefining, 5(5), pp.562–
569.
Kumar, R., Tabatabaei, M., Karimi, K., Horváth, I.S.
2016. Recent updates on lignocellulosic biomass
derived ethanol -A review. Biofuel Research Journal,
9(9), pp.347–356.
Lam, F.H., Ghaderi, A., Fink, G. R., Stephanopoulos, G.
2014. Engineering alcohol tolerance in yeast. Science,
346(6205), pp.71–5. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/25278607
[Accessed March 28, 2017].
Lee, F. M., Pahl, R. H., 1985. Use of gasoline to extract
ethanol from aqueous solution for producing gasohol.
Industrial and Engineering Chemistry Process Design
and Development, 24(2), pp.250–255. Available at:
http://pubs.acs.org/doi/abs/10.1021/i200029a005
[Accessed November 30, 2017].
Leeper, S. A., Wankat, P. C., 1982. Gasohol production by
extraction of ethanol from water using gasoline as
solvent. Industrial and Engineering Chemistry
Process Design and Development, 21(2), pp.331–334.
Available at:
http://pubs.acs.org/doi/abs/10.1021/i200017a018
[Accessed November 30, 2017].
Li, Y. J., Lu, Y. Y., Zhang, Z. J., Mei, S., Tan, T. W., Fan
L. H. 2017. Co-fermentation of cellulose and
sucrose/xylose by engineered yeasts for bioethanol
production. Energy & Fuels,
p.acs.energyfuels.7b00032. Available at:
http://pubs.acs.org/doi/abs/10.1021/acs.energyfuels.7b
00032 [Accessed March 30, 2017].
Olofsson, J., Barta, Z., Börjesson, P. and Wallberg, O.
2017. Integrating enzyme fermentation in
lignocellulosic ethanol production: life-cycle
assessment and techno-economic analysis.
Biotechnology for Biofuels, 10(1), p.51. Available at:
http://biotechnologyforbiofuels.biomedcentral.com/art
icles/10.1186/s13068-017-0733-0.
Pertamina. 2017. Spesifikasi Pertamax, Available at:
http://www.pertamina.com/industrialfuel/media/24240
/pertamax.pdf [Accessed November 29, 2017].
Singh, N., Mathur, A.S., Tuli, D.K., Gupta, R.P., Barrow,
C.J., Puri, M. 2017. Cellulosic ethanol production via
consolidated bioprocessing by a novel thermophilic
anaerobic bacterium isolated from a Himalayan hot
spring. Biotechnology for Biofuels, 10(1), p.73.
Available at:
http://biotechnologyforbiofuels.biomedcentral.com/art
icles/10.1186/s13068-017-0756-6 [Accessed March
30, 2017].
U.S. EIA. 2016. International Energy Outlook 2016,
Available at: http://www.hm-
treasury.gov.uk/independent_reviews/stern_review_ec
onomics_climate_change/stern_review_report.cfm.
Vohra, M., Manwar, J., Manmode, R., Padgilwar, S. and
Patil, S. 2014. Bioethanol production: Feedstock and
current technologies. Journal of Environmental
Chemical Engineering, 2(1), pp.573–584.
Xu, Y. and Wang, D. 2017. Integrating starchy substrate
into cellulosic ethanol production to boost ethanol
titers and yields. Applied Energy, 195, pp.196–203.
Available at:
http://www.sciencedirect.com/science/article/pii/S030
6261917302593 [Accessed March 30, 2017].
Ziolkowska, J. R. 2014. Prospective technologies,
feedstocks and market innovations for ethanol and
biodiesel production in the US. Biotechnology
Reports, 4, pp.94–98.
Influence of Ions and Temperatures on Water-Ethanol-Gasoline Liquid-Liquid Equilibrium
103
