
Difference Triterpenoid and Phytosterol Profile between Kandelia
candel and K. obovata
Mohammad Basyuni
1,2
, Shigeyuki Baba
3
, Hirosuke Oku
4
, Fairus Mulia
5
, Yuntha Bimantara
1
,
Sumaiyah
2,6
and Era Yusraini
2,7
1
Department of Forestry, Faculty of Forestry, Universitas Sumatera Utara, Jl. Tri Dharma Ujung No. 1 Medan, North
Sumatera 20155, Indonesia
2
Center of Excellence for Mangrove, Universitas Sumatera Utara, Medan, North Sumatera 20155, Indonesia
3
International Society for Mangrove Ecosystems, Faculty of Agriculture, University of the Ryukyus, 1 Senbaru, Nishihara,
Okinawa 903-0213, Japan
4
Molecular Biotechnology Group, Tropical Biosphere Research Center, University of the Ryukyus, 1 Senbaru, Nishihara,
Okinawa 903-0213, Japan
5
PT. Kandelia Alam, Kubu Raya District, West Kalimantan, Indonesia
6
Faculty of Pharmacy, Universitas Sumatera Utara, Medan 20155, Indonesia
7
Faculty of Agriculture, Universitas Sumatera Utara, Medan 20155, Indonesia
fairus.mulia@live.com, sumaiyah7777@gmail.com, era_yusraini@yahoo.com
Keywords: Isoprenoid Composition, Kandelia candel, K. obovata, True Mangrove.
Abstract: Kandelia, a genus belonging to Rhizophoraceae has been reported to have two distinct species: K. candel and
K. obovata. Mangrove plants are known to produce secondary metabolite mostly derived from isoprenoid
(triterpenoid and phytosterol). Isoprenoid composition of leaves and roots of K. candel (L.) Druce and K.
obovata Sheue, Liu & Yong were investigated and compared. Triterpenoid and phytosterol profile of both
species was analyzed using Gas Chromatography with Flame Ionization Detector (GC-FID). Both species
displayed difference composition either in the leaves or roots. In the leaves of K. candel, eight isoprenoids
detected, with dominating of -amyrin, a member of triterpenoid. The ratio between triterpenoid and
phytosterol was 73.2%:26.8%. By contrast, phytosterol dominated the isoprenoid proportional in the roots of
K. candel (91.7%). Similar results were found in the K. obovata leaves and roots, a predominated phytosterol
over triterpenoid, 59.6%, and 97.9%, respectively. The present work suggested diversity
composition of isoprenoid in both Kandelia.
1 INTRODUCTION
Mangroves are known to produce triterpenoids and
phytosterols (or called isoprenoids) (Volkman 2005;
Basyuni et al., 2007), as well as the cases for other
many plant species (Yendo et al., 2010; Thimmappa
et al., 2014). These tree species are not only a source
of genes encoding enzymes in the triterpenes and
phytosterol biosynthetic pathways but also potential
plants that may have prospective medicinal and
agricultural value (Sari et al., 2018). These mangrove
characteristics may expose another prospect of
mangrove use.
A number of reports have involved triterpenoids
as compatible evidence for the main origin of organic
matter from mangrove due to their immovability
during sedimentation and diagenesis (Killops and
Frewin, 1994; Versteegh et al., 2004; Koch et al.,
2011).
Because of their varied array of biological
properties, isoprenoids are recognized as necessary as
pure prospective sources for medicinal activities.
Several biological activities have been described for
triterpenes: anti-inflammatory activity for taraxerol,
-amyrin, -amyrin, lupeol and germanicol (Kim et
al. 2005; Melo et al., 2011); anti-carcinogenic activity
for taraxerol and germanicol (Jang et al. 2004);
insecticidal property for taraxerol (William, 1999),
cardioprotective impact in hypercholesterolemic
syndrome for lupeol (Sudhahar et al., 2007),
hepatoprotective counter to acetaminophen-induced
hepatotoxicity for - and -amyrin (Olievera et al.,
Basyuni, M., Baba, S., Oku, H., Mulia, F., Bimantara, Y., Sumaiyah, . and Yusraini, E.
Difference Triterpenoid and Phytosterol Profile between Kandelia candel and K. obovata.
DOI: 10.5220/0008505400510054
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 51-54
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
51
2005), Stigmasterol, plentifully available in Acanthus
illicifolius, has been shown to possess
hypercholesterolemic cases (Kokpol et al., 1986),
antimicrobial activity for polyisoprenoid (Sumardi et
al., 2018), anticancer colon property for
polyisoprenoid including dolichol (Illian et al., 2018;
Sari et al. 2018a,b).
Triterpenes are mostly accumulated in plants as
their glycosides and saponins. In order to get more
insight into biological and medicinal functions of
triterpenoid and phytosterol in mangrove plants,
Kandelia candel, and K. obovata, it is; therefore, the
study is aimed to analyze the composition of
isoprenoid. Here we report the different composition
of triterpenoid and phytosterol of Kandelia candel
and K. obovata leaves and roots.
2 MATERIALS AND METHOD
Kandelia obovata leaves and roots were collected
from Okukubi River, Okinawa, Japan. Leaves and
roots of K. obovata were obtained from PT. Kandelia
Alam, Kubu Raya District, West Kalimantan,
Indonesia. These materials were taken directly into
dry ice and stored at –20 ºC for further investigation.
The leaves or roots (5 g wet weight,
correspondingly) were first crushed in liquid nitrogen
and extracted with chloroform-methanol (2:1 by
volume) (CM21). The cell wall debris insoluble in
CM21 was discarded by filtration through No. 2 filter
paper (Advantec, Tokyo, Japan), and the extract was
incompletely purified for lipid analysis as reported
previously (Basyuni et al., 2007).
The lipid extract consists of 2 mg of total lipid,
then saponified at 60 ºC for 24h with 3% KOH in 94%
ethanol. The nonsaponifiable lipids (NSL) separated
into hexane by robust infuse were determined by gas
chromatography (GC 2010). The column used was
CBP1-M50-025 (Shimadzu), and the column
temperature was set up from 1 min hold at 50 ºC to a
final temperature of 300 ºC at a rate of 10 ºC/min as
earlier described (Basyuni et al., 2007, 2012).
3 RESULTS
Table 1 shows the triterpenoid and phytosterol
composition of K. candel leaves and roots. In the
leaves, -amyrin, a member of triterpenoid has the
most significant proportion (38.5%), then followed
by lupeol and -amyrin. By contrast, in the roots of
K. candel, stigmasterol (49.6%), a member of
phytosterols predominated over triterpenoids (91.7%:
8.3% in ratio). This finding was supported by
previous results on the tree of K. candel leaves with
major components was -amyrin (45.2%) and -
amyrin (18.0%) (Basyuni et al. 2007). Similarly, in
the roots of K. candel tree, the essential compounds
were phytosterols (-sitosterol, stigmasterol, and
campesterol) (Basyuni et al., 2007).
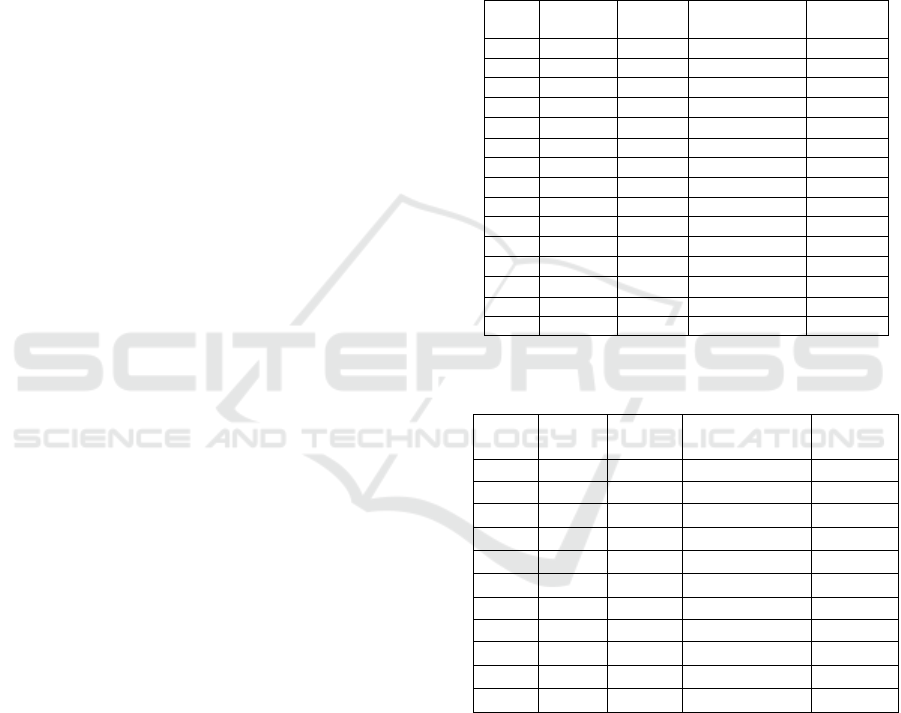
Table 1: Triterpenoid and phytosterol composition in K.
candel leaves and roots.
Tissue
RT (min)
Area
Compound
Proportion
(%)
41.490
2507.3
Campesterol (1)
2.6±0.2
42.395
24252.7
Stigmasterol (2)
4.9±0.8
44.285
69363.8
-sitosterol (3)
14.8±0.6
Leaves
45.072
7228.1
Lanosterol (4)
1.8±0.4
46.864
180485.3
-amyrin (5)
38.5±0.8
46.864
10812.2
Cycloartenol (6)
2.7±0.4
47.122
100902.4
Lupeol (7)
21.3±0.5
47.334
60279.2
-amyrin (8)
13.5±0.2
41.490
2507.3
Campesterol (1)
18.2±1.1
42.395
24252.7
Stigmasterol (2)
49.6±0.8
Roots
44.285
69363.8
-sitosterol (3)
20.3±3.9
45.072
7228.1
Lanosterol (4)
3.7±1.8
45.752
180485.3
-amyrin (5)
2.8±0.9
46.864
10812.2
Cycloartenol (6)
3.2±0.4
47.122
100902.4
Lupeol (7)
2.3±0.5
Table 2: Triterpenoid and phytosterol composition in K.
obovata leaves and roots.
Tissue
RT (min)
Area
Compound
Proportion
(%)
41.750
32959.8
Campesterol (1)
6.3±0.8
42.661
81775.6
Stigmasterol (2)
18.3±1.7
44.580
146898.3
-sitosterol (3)
33.5±3.8
Leaves
45.655
8322.1
Lanosterol (4)
1.4±0.5
46.031
88652.9
-amyrin (5)
17.4±1.6
46.450
9037.7
Lupenone (6)
2.0±0.1
47.432
108905.9
Lupeol (7)
21.0±2.6
41.750
2507.3
Campesterol (1)
16.6±0.7
42.666
24252.7
Stigmasterol (2)
46.8±0.5
Roots
44.565
69363.8
-sitosterol (3)
34.5±0.2
45.800
180485.3
-amyrin (4)
2.1±0.4
Table 2 compiles the isoprenoid profile in the
leaves and roots of K. obovata. The isoprenoid profile
of K. obovata leaves was not similar compared K.
candel leaves. Phytosterols dominated over
triterpenoids in the leaves. However, in case of roots
were rather similar, phytosterols were the main
components of both K. obovata and K. candel (Tables
1 and 2).
In the seedlings stage of K. candel leaves, the main
components were -amyrin, lupeol and -amyrin,
while the phytosterols were minor composition
ICONART 2019 - International Conference on Natural Resources and Technology
52
(Basyuni et al., 2009). Parallel with the results,
phytosterols especially stigmasterol was detected to
be the dominant compound in the roots of K. candel
seedlings (Basyuni et al., 2009, 2012).
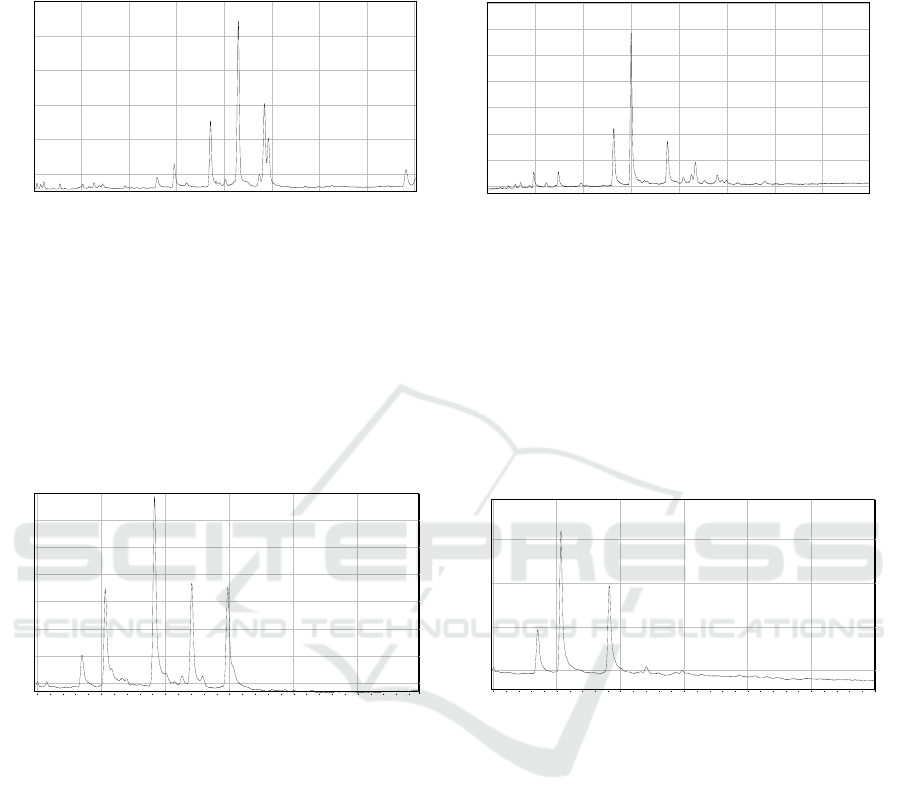
Figure 1: GC-FID profile of K. candel leaves and roots. For the compound name, please refer to Table 1.
Furthermore, a bit different composition was
shown by KcMS multifunctional triterpene synthase
from K. candel. Lupeol had 50% proportion then
followed by -amyrin and -amyrin (25%) (Basyuni
et al., 2006). The occurrence of triterpenoids (lupeol,
-amyrin, and -amyrin) as previously reported for
fatty acid esters in leaves and roots of K. candel
seedlings (Oku et al., 2003).
Figure 1 and 2 depict the GC-FID profile of K.
candel and K. obovata leaves and roots. The
identification of triterpenoid and phytosterol in the
GC profile mainly by the analogy of their retention
time on the GC column with those authentic standards
and analysis of the mass spectrum (Basyuni et al.,
2007, 2012).
Figure 2: GC-FID profile of K. obovata leaves and roots. For the compound name, please refer to Table 2.
4 CONCLUSIONS
Difference isoprenoids composition between K.
candel and K. obovata leaves and roots have been
confirmed in this study. In the leaves of K. candel, the
dominating of -amyrin, a member of triterpenoid
with contrast to the roots, phytosterol dominated the
isoprenoid proportional. K. obovata leaves and roots,
a predominated phytosterol over triterpenoid was
found. The present work suggested diversity
composition of isoprenoid in both Kandelia.
ACKNOWLEDGMENTS
We thank the Universitas Sumatera Utara for an
International Research Collaboration and Scientific
Publication Grant 2019.
REFERENCES
Basyuni, M., Oku, H., Inafuku, M., Baba, S., Iwasaki, H.,
Oshiro, K., Shibuya, Y., and Ebizuka, Y. 2006.
Molecular cloning and functional expression of a
multifunctional triterpene synthase cDNA from a
mangrove species Kandelia candel (L.) Druce.
Phytochemistry, 67(23), 2517-2524.
35.0 37.5 40.0 42.5 45.0 47.5 50.0 52.5
min
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
ƒÊV(x1,000)
0.0
25.0
50.0
75.0
Ž
K. candel roots
7
6
5
4
3
2
1
37.5 40.0 42.5 45.0 47.5 50.0 52.5
min
0.5
1.0
1.5
2.0
2.5
3.0
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. candel leaves
1
3
4
5
6
7
8
2
35.0 37.5 40.0 42.5 45.0 47.5 50.0 52.5
min
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
ƒÊV(x1,000)
0.0
25.0
50.0
75.0
Ž
K. candel roots
7
6
5
4
3
2
1
37.5 40.0 42.5 45.0 47.5 50.0 52.5
min
0.5
1.0
1.5
2.0
2.5
3.0
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. candel leaves
1
3
4
5
6
7
8
2
40.0 42.5 45.0 47.5 50.0 52.5
min
0.5
1.0
1.5
2.0
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. obovata roots
4
3
2
1
40.0 42.5 45.0 47.5 50.0 52.5
min
0.50
0.75
1.00
1.25
1.50
1.75
2.00
2.25
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. obovata leaves
5
6
7
1
2
3
4
40.0 42.5 45.0 47.5 50.0 52.5
min
0.5
1.0
1.5
2.0
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. obovata roots
4
3
2
1
40.0 42.5 45.0 47.5 50.0 52.5
min
0.50
0.75
1.00
1.25
1.50
1.75
2.00
2.25
ƒÊV(x10,000)
0.0
25.0
50.0
75.0
Ž
K. obovata leaves
5
6
7
1
2
3
4
Difference Triterpenoid and Phytosterol Profile between Kandelia candel and K. obovata
53

Basyuni, M., Oku, H., Baba, S., Takara, K. and Iwasaki, H.,
2007. Isoprenoids of Okinawan mangroves as lipid
input into estuarine ecosystem. Journal of
Oceanography, 63(4), 601-608.
Basyuni, M., Baba, S., Inafuku, M., Iwasaki, H., Kinjo, K.,
and Oku, H. 2009. Expression of terpenoid synthase
mRNA and terpenoid content in salt stressed mangrove.
Journal of Plant Physiology, 166(16), 1786-1800.
Basyuni, M., Baba, S., Kinjo, Y., Putri, L. A., Hakim, L.
and Oku, H., 2012. Salt-dependent increase in
triterpenoids is reversible upon transfer to fresh water
in mangrove plants Kandelia candel and Bruguiera
gymnorrhiza. Journal of Plant Physiology, 169(18),
1903-1908.
Illian, D. N., Basyuni, M., Wati, R., & Hasibuan, P. A. Z.
2018. Polyisoprenoids from Avicennia marina and
Avicennia lanata inhibit WiDr cells proliferation.
Pharmacognosy Magazine, 14(58), 513-518.
Jang, D. S., Cuendet, M., Pawlus, A. D., Kardono, L. B. S.,
Kawanishi, K., Farnsworth, N. R., Fong, H. H. S.,
Pezzuto, J. M., Kinghorn, A. D., 2004. Potential cancer
chemopreventive constituents of the leaves of
Macaranga triloba. Phytochemistry 65, 345-350.
Killops, S. D., Frewin, N. L., 1994. Triterpenoid diagenesis
and cuticular preservation. Organic Geochemistry 21,
1193-1209.
Kim, D. K., Lim, J. P., Kim, J. W., Park, H. W. and Eun,
J.S., 2005. Antitumor and antiinflammatory
constituents fromceltis sinensis. Archives of
Pharmacal Research, 28(1), 39-43.
Melo, C. M., Morais, T. C., Tomé, A. R., Brito, G. A. C.,
Chaves, M. H., Rao, V. S., & Santos, F. A. 2011. Anti-
inflammatory effect of α, β-amyrin, a triterpene from
Protium heptaphyllum, on cerulein-induced acute
pancreatitis in mice. Inflammation research, 60(7),
673-681.
Oku, H., Baba, S., Koga, H., Takara, K., and Iwasaki, H.
2003. Lipid composition of mangrove and its relevance
to salt tolerance. Journal of Plant Research, 116(1), 37-
45.
Oliveira, F. A., Chaves, M. H., Almeida, F. R. C., Lima Jr.,
R. C. P., Silva, R. M., Maia, J. L., Brito, G. A. A. C.,
Santos, F. A. and Rao, V. S., 2005. Protective effect of
- and -amyrin, a triterpene mixture from Protium
heptaphyllum (Aubl.) March. trunk wood resin, against
acetaminophen-induced liver injury in mice. Journal of
Ethnopharmacology 98, 103-108.
Sari, D. P., Basyuni, M., Hasibuan, P. A., Sumardi, S.,
Nuryawan, A. and Wati, R., 2018a. Cytotoxic and
Antiproliferative Activity of Polyisoprenoids in
Seventeen Mangroves Species Against WiDr Colon
Cancer Cells. Asian Pacific Journal of Cancer
Prevention, 19 (12), 3393-3400.
Sari, D. P., Basyuni, M., Hasibuan, P. A. Z., Wati, R., and
Sumardi. 2018b. Cytotoxic effect of polyisoprenoids
from Rhizophora mucronata and Ceriops tagal leaves
against WiDr colon cancer cell lines. Sains Malaysiana,
47(9), 1953-1959.
Sudhahar, V., Kumar, S. A., Sudharsan, P. T. and
Varalakshmi, P., 2007. Protective effect of lupeol and
its ester on cardiac abnormalities in experimental
hypercholesterolemia. Vascular Pharmacology, 46(6),
412-418.
Sumardi, S., Basyuni, M. and Wati, R., 2018. Antimicrobial
activity of polyisoprenoids of sixteen mangrove species
from North Sumatra, Indonesia. Biodiversitas Journal
of Biological Diversity, 19(4), pp.1243-1248.
Thimmappa, R., Geisler, K., Louveau, T., O'Maille, P., and
Osbourn, A. 2014. Triterpene biosynthesis in plants.
Annual Review of Plant Biology, 65, 225-257.
Versteegh, G. J., Schefuß, E., Dupont, L., Marret, F.,
Damsté, J. S. S. and Jansen, J. F., 2004. Taraxerol and
Rhizophora pollen as proxies for tracking past
mangrove ecosystems. Geochimica et Cosmochimica
Acta, 68(3), 411-422.
Volkman, J. K., 2005. Sterols and other triterpenoids:
source specificity and evolution of biosynthetic
pathways. Organic Geochemistry, 36(2),139-159.
Williams, L. A. D., 1999. Rhizophora mangle
(Rhizophoraceae) triterpenoids with insecticidal
activity. Naturwissenschaften 86, 450-452.
Yendo, A. C., de Costa, F., Gosmann, G., and Fett-Neto, A.
G. 2010. Production of plant bioactive triterpenoid
saponins: elicitation strategies and target genes to
improve yields. Molecular Biotechnology, 46(1), 94-
104.
ICONART 2019 - International Conference on Natural Resources and Technology
54
