
Plant Polyprenol Reductase in the Database
Mohammad Basyuni
1,2
, Rahmah Hayati
1
, Yuntha Bimantara
1
, Arif Nuryawan
1
, Etti Sartina Siregar
3
and Sumaiyah
4
1
Department of Forestry, Faculty of Forestry, Universitas Sumatera Utara, Jl. Tri Dharma Ujung No. 1 Medan, North
Sumatra 20155, Indonesia
2
Mangrove and Bio-Resources Group, Center of Excellence for Natural Resources Based Technology, Universitas
Sumatera Utara, Medan North Sumatra 20155, Indonesia
3
Faculty of Mathematics and Natural Sciences, Universitas Sumatera Utara, Medan 20155, Indonesia
4
Faculty of Pharmacy, Universitas Sumatera Utara, Medan 20155, Indonesia
Keywords: Biotechnology, gene, plasma membrane, polyprenol reductase,
Abstract: Chemical constituents such as polyprenols and dolichols are known to generate higher plants. The present
document discusses the database search (https:/www.ncbi.nlm.nih.gov/) or GQuery on plant polyprenol
reductase from the National Center for Biotechnology Information (NCBI). A amount of useful data was
searched for NCBI databases. Results developed for plant polyprenol reductase in 10 databases. All
polyprenol reductase plant databases consisting of source literature, genes, genetics, protein, genomes, and
chemical characteristics. It is noteworthy that plant polyprenol reductase has complete information. The
literature comprised of PubMed and PubMed Central. Gene consisted of Gene, GEO Profiles, and UniGene.
Genetics data was available for plant polyprenol reductases such as dbGap and MedGen. Proteins feature
contained four protein sequences. Genomes encompassed one BioSample and 365 Nucleotides. The
chemicals property denoted 3392 BioSystems. The present study affords indispensable information
concerning biotechnology of plant polyprenol reductase.
1 INTRODUCTION
Higher plants are well known to produce short to
lengthy polyisoprenoids, which are classified into
polyprenol and dolichol (Sagami et al., 2018). The
occurrence of polyisoprenoids in the tropical,
subtropical and temperate areas was outlined in
different plant organs (Skorupins-Tudek et al., 2008;
Surmacz and Swiezewska, 2011; Basyuni et al.,
2016, 2017 Arifiyanto et al., 2017; Basyuni and
Wati, 2017). These reports demonstrated the
extensive distribution in the plant kingdom of
polyisoprenoids. In plant leaves, polyprenols are
defined and minor dolichols are identified
(Skorupins-Tudek et al., 2008; Surmacz and
Swiezewska, 2011; Arifiyanto et al., 2017; Basyuni
and Wati, 2017). Otherwise, dolichols in plant roots,
yeast and animal tissues were identified extensively
(Grabińska. and Palamarczyk 2002; Ishiguro et al.,
2014; Basyuni et al., 2016, 2017; Sagami et al.,
2018). By contrast to these studies, it is noteworthy
that the primary structure of polyisoprenoids in
mangroves and coastal plant leaves and roots was
dolichols (Basyuni et al., 2016, 2017).
The presence of adequate dolichols in these
plants leaves suggests that the polyprenol reductase
enzyme worked (Basyuni et al., 2018a,b,c). It has
also been reported the presence of polyprenol
reductase enzyme in Arabidopsis thaliana (Jozwiak
et al., 2015) and other higher plants (Basyuni and
Wati, 2018). This enzyme connected with the
SRD5A3 protein in animals has been revealed to
catalyze the final step in the dolichol biosynthetic of
polyprenol converted to dolichols (Rosenwald et al.,
1993; Cantagrel et al., 2010; Gründahl et al., 2012;
Sagami et al., 2018). Limited work focused on the
biotechnology data investigated from all earlier
accessible NCBI databases in the plant polyprenol
reductase. Here, through a search engine, we report a
quick and simple technique to collect useful data
needed in latest biotechnology-related science
studies. The aim of this research is to describe the
implementation of the National Center for
Biotechnology Information (NCBI) databases in
12
Basyuni, M., Hayati, R., Bimantara, Y., Nuryawan, A., Siregar, E. and Sumaiyah, .
Plant Polyprenol Reductase in the Database.
DOI: 10.5220/0008386700120015
In Proceedings of the International Conference on Natural Resources and Technology (ICONART 2019), pages 12-15
ISBN: 978-989-758-404-6
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
order to obtain more insight into useful data on
updated biotechnology on polyprenol reductase in
plants.
2 MATERIAL AND METHOD
The online search engine for NCBI databases
(https:/www.ncbi.nlm.nih.gov/) has been used to
produce useful data about polyprenol reductase in
the plant. As stated previously on 19 February 2018,
databases were accessed by typing plant and
polyprenol reductase in all NCBI databases. The
characteristic was all databases made using GQuery
that consisted of the literature, genes, protein,
genomes and chemical characteristics of plant
polyprenol reductase. The considerations for data
included the PubMed, PubMed Central, GEO (Gene
Expression Omnibus) profiles, UniGene, dbGaP
(database of Genotypes and Phenotypes), MedGen,
Protein, Assembly, BioCollections, BioSamples,
Clone, GSS (Genome Survey Sequences),
Nucleotide, and BioSystems.
3 RESULTS AND DISCUSSION
The search for plant polyprenol reductase led ten
databases for plant polyprenol reductase in the NCBI
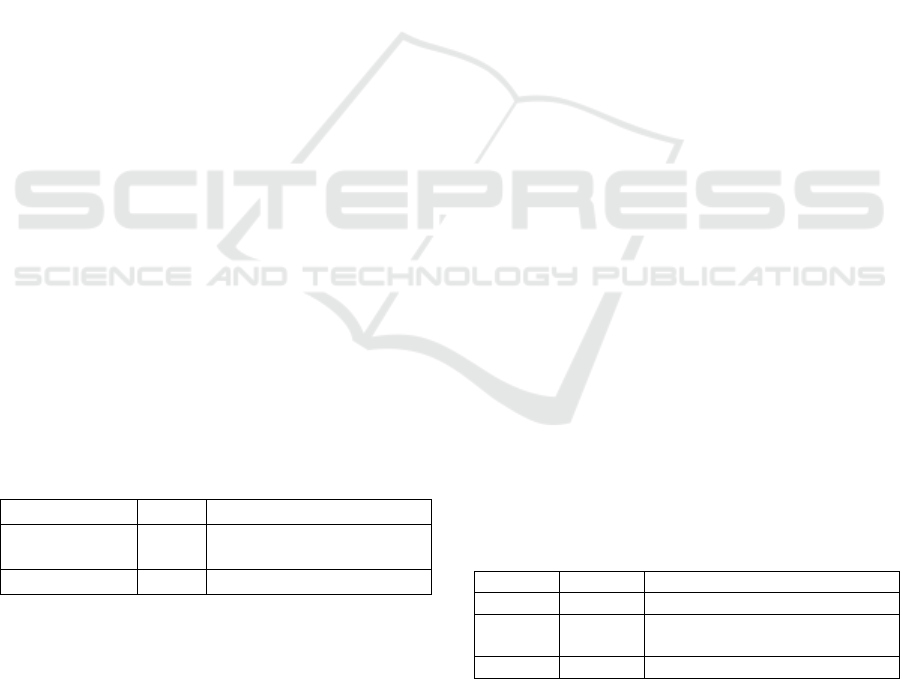
database. Table 1 demonstrates the literature on
plant polyprenol reductase available in the NCBI.
Two biographies deposited with countless figures.
The online NCBI literature provides online libraries
and free access to two PubMed papers consisting of
science and medical abstracts / citations and 23
PubMed Central articles.
Table 1: Literature source NCBI database for plant and
polyprenol reductase
Literature
Number
Description
PubMed
2
Abstracts/quotations from
science and medicine
PubMed Central
23
Articles in full-text journals
Information on sources of genes is shown in
Table 2. This information includes plant-related
polyprenol reductase genes than consisting of one
gene, namely TNF receptor-associated factor 6
[Harpegnathos saltator (Jerdon's jumping ant)].
Table 2 shows 20 GEO profiles and three unigenes.
Arabidopsis thaliana species dominated the Geo
profiles. The Geo profiles are AT1G72590-Red light
effect on the root, AT2G16530-Diurnal and
circadian-regulated genes (II), AT1G72590-
Necrosis- and ethylene-inducing peptide effect on
dicots, AT1G72590-Leaf Response to Aphid
Feeding, AT1G72590-MicroRNA miR159a
overexpression effect on flower, AT1G72590-
MicroRNA miR319a overexpression effect on the
leaf, AT1G72590-Vier F-Box VFB triple mutant
seedlings, AT1G72590-Chitin oligomer chitooctaose
effect on seedlings, the effect of AT1G72590-
MicroRNA miR156b and miR164b on floral apex,
AT1G72590- MicroRNA miR164b and miR172a on
vegetative apex, DFG10-Saponin treatment effect:
time course, AT2G16530-Indole acetic acid
treatment time course (AG), AT1G72590- the
impact of salt stress on mannitol-producing M6PR
transgenic plant with salt tolerance: leaf,
AT1G72590-Indole acetic acid treatment time
course (ATH1), AT1G72590- mRNA cap-binding
protein ABH1 mutant response to abscisic acid,
AT1G72590-Salt stress effect on multiple
genotypes: leaf, LOC4336740-Cytokinin effect on
rice roots and leaves: time course, AT1G72590
Ozone impact on double-null mutant leaves of G-
protein: time span, AT1G72590-Pollinated pistils:
time span, and AT1G72590-Indole acetic acid
therapy: time span and time span (Table 2).
The UniGene consisted of 3 clusters of expressed
transcripts. They are Putative polyprenol reductase 2
(AT2G16530), represented by 11 ESTs from 5
cDNA libraries. This gene corresponds to 2
reference sequences (different isoforms) (Joswiak et
al., 2015). The 2
nd
UniGene was Probable polyprenol
reductase 2-like, Soybean protein-encoding gene
LOC100814660. Displayed by 13 ESTs from 8
cDNA libraries and Transcribed locus, moderately
similar to XP_003537828.1 predicted: probable
polyprenol reductase 2-like Glycine max. This
unigene was represented by Lotus japonicus putative
protein-coding gene and represented by 4 ESTs from
3 cDNA libraries.
Table 2: Genes source NCBI database for plant polyprenol
reductase
Genes
Total
Information
Gene
1
Collected gene loci data
GEO
Profiles
20
Gene expression and profiles of
molecular abundance
UniGene
3
Expressed transcript clusters
Furthermore, the genetics source NCBI database
comprises of 8 dbGaP and 2 MedGen. The study of
the database of Genotypes and Phenotypes consist of
phs000287.v6.p1 Cardiovascular Health Study
(CHS) Cohort, phs000280.v5.p1 Atherosclerosis
Plant Polyprenol Reductase in the Database
13
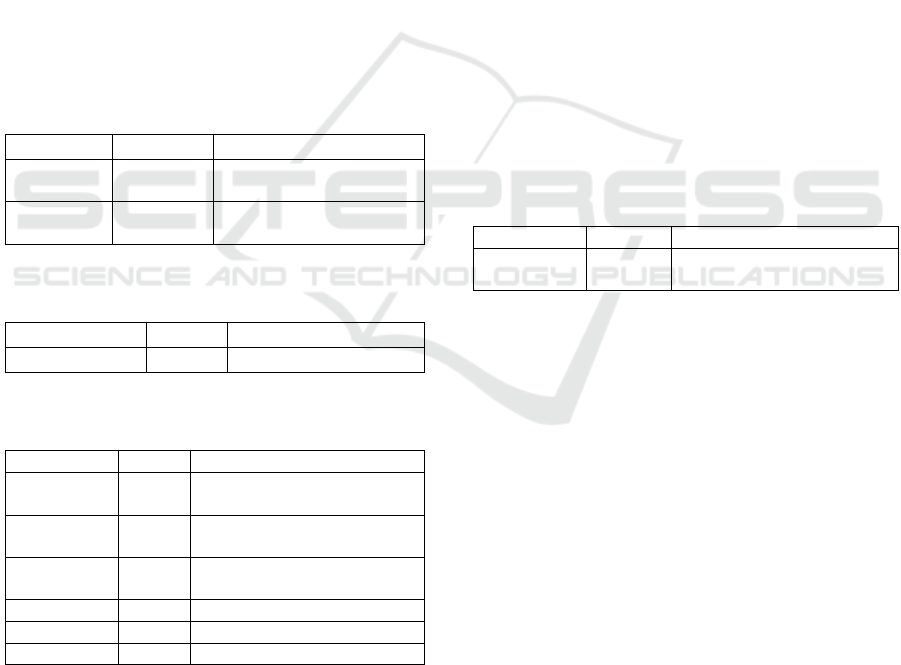
Risk in Communities (ARIC) Cohort,
phs000209.v13.p3 Multi-Ethnic Study of
Atherosclerosis (MESA) Cohort, phs001496.v1.p1
A genomic approach to the prescription of warfarin
in Hispanics of the Admixed Caribbean,
phs000007.v30.p11 Framingham Cohort,
phs000309.v3.p2 The CARDIA-GENEVA Study,
phs000090.v5.p1 GENEVA: The Atherosclerosis
Risk in Communities (ARIC) Study, and
phs000547.v1.p1 A Study of the Genome-Wide
Association in SUCCESS Trial Breast Cancer
Patients. Regarding with protein for plant polyprenol
reductase observed in the database, 55 proteins were
recorded.
The biological and pharmacological function of
polyisoprenoids especially dolichols have been well
documented (Tao et al., 2016; Illian et al., 2018; Sari
et al., 2018). Furthermore, the role of dolichols for
the clinical and biochemical phenotypes in dolichol-
linked Congenital disorders of glycosylation (CDG)
has been described (Buczkowska et al., 2015).
Table 3: Genetics source NCBI database for plant and
polyprenol reductase
Genetics
Number
Explanation
dbGaP
8
Genotype/phenotype
interaction studies
MedGen
2
Medicinal genetics
literature and links
Table 4: Protein source NCBI database and plant
polyprenol reductase
Proteins
Number
Description
Protein
55
Protein sequence
Table 5: Genomes source NCBI database for plant
polyprenol reductase
Genomes
Number
Information
Assembly
24
Information on assembly of
genomes
BioCollections
149
Museum, herbarium and other
collections of biorepository
BioSample
1
Information of of materials
from biological sources
Clone
12
cDNA and genomic clones
GSS
2
Genome search sequences
Nucleotide
266
RNA and DNA sequences
The MedGene has two kinds of Glycyrrhetinic
acid product and High-Selenium Brassica juncea
(Table 3). Table 4 shows 55 protein sequences that
consist of 39 eudicots and 16 monocots. The top
plant species for protein sequence in the plant
polyprenol reductases are Noccaea caerulescens
(12), Vitis vinifera (6), Arachis ipaensis (6), Ananas
comosus (5), Dendrobium catenatum (5), and
Dichanthelium oligosanthes (3), and others (18).
Furthermore, it has been shown from Table 5;
there are 266 nucleotides from plant polyprenol
reductase, which classified from molecular type as
227 from genomic/RNA and only 22 from mRNA.
While from the sources database of nucleotide are
derived from INSDC (International Nucleotide
Sequence Database Collaboration)/Genbank (109)
and RefSeq (157). The mRNA molecule type of
plant polyprenol reductase is derived from Arachis
ipaensis (6), Ananas comosus (5), Dendrobium
catenatum (4), Arachis duranensis (2). Juglans regia
(2), and all other taxa (3).
Recently three predicted polyprenol reductases
from K. obovata had been described (Basyuni et al.,
2018a,b,c) to add previous information on
polyprenol reductase from A. thaliana (Jozwiak et
al., 2015). The position of predicted polyprenol
reductase K. obovata existed together with Ricinus
communis and Iopoea nil (Basyuni et al., 2018b,c;
Basyuni and Wati, 2018). Polyprenol reductase also
occurred in yeast Saccharomyces cerevisiae
(Szkopinska et al., 2006).
Table 6: Chemicals source NCBI database for plant and
polyprenol reductase
Chemicals
Total
Explanation
BioSystems
3392
Gene, protein and chemicals
linked molecular pathways
No variation of chemical features of the plant
polyprenol reductase as was displayed in Table 5, in
this report only BioSystems were detected.
Biosystems contained two types, conserved
biosystems (135) and organism-specific biosystems
(3257). According to a record type, 3,359 pathways,
28 structural complexes, five working sets.
Concerning to source name consisting of GO (135)
and KEGG (3,255). The BioAssays comprised
BioAssays via Actives (3,255) and BioAssays via
Target (157).
ICONART 2019 - International Conference on Natural Resources and Technology
14

4 CONCLUSIONS
The web NCBI supplied countless data on plant
polyprenol reductase in biology and biotechnology.
The current research encouraged researchers in the
field of biotechnology to obtain more advantages
using the NCBI search engine. The present study
also delivers crucial data regarding biotechnology of
plant polyprenol reductase.
ACKNOWLEDGMENTS
World Class Research asissted this work from
Directorate for Research and Community Service,
Ministry of Research, Technology and Higher
Education, the Republic of Indonesia.
REFERENCES
Arifiyanto, D., Basyuni, M., Sumardi, S., Putri, L.A.P.,
Siregar, E.S., Risnasari, I. and Syahputra, I., 2017.
Occurrence and cluster analysis of palm oil (Elaeis
guineensis) fruit type using two-dimensional thin layer
chromatography. Biodiversitas Journal of Biological
Diversity, 18(4), 1487-1492.
Basyuni, M., Sagami, H., Baba, S., Iwasaki, H. and Oku,
H., 2016. Diversity of polyisoprenoids in ten
Okinawan mangroves. Dendrobiology, 75. 167-175.
Basyuni, M., Sagami, H., Baba, S., Putri, L.A., Wati, R.
and Oku, H., 2017. Salinity Alters the Polyisoprenoid
Alcohol Content and Composition of Both Salt-
Secreting and Non–Salt-Secreting Mangrove
Seedlings. HAYATI Journal of Biosciences, 24(4),
206-214.
Basyuni, M. and Wati, R., 2017. Distribution and
occurrence of polyisoprenoids in rambutan
(Nephellium lappaceum). Conference Series: Earth
and Environmental Science 101, 012001.
Basyuni, M., Sagami, H., Baba, S., and Oku, H. 2018a.
Genome sequence analysis of predicted polyprenol
reductase gene from mangrove plant Kandelia
obovata. IOP Conference Series: Earth and
Environmental Science, 130, 012039.
Basyuni, M., Wati, R., Sagami, H., Oku, H., and Baba, S.
2018b. Bioinformatics approach of three partial
polyprenol reductase genes in Kandelia obovata.
Journal of Physics: Conference Series, 978, 012044.
Basyuni, M., Baba, S., Wati, R., Sumardi, Sulistiyono, N.,
Oku, H., and Sagami, H. 2018. Isolation and
phylogenetic analysis of new predicted polyprenol
reductase from mangrove plant (Kandelia obovata
Sheue, HY Liu & J. Yong). In AIP Conference
Proceedings 2002, 020041.
Basyuni, M., and Wati, R. 2018. Bioinformatics analysis
of the predicted polyprenol reductase genes in higher
plants. Journal of Physics: Conference Series, 978,
012050.
Buczkowska, A., Swiezewska, E., and Lefeber, D. J. 2015.
Genetic defects in dolichol metabolism. Journal of
Inherited Metabolic Disease, 38(1), 157-169.
Cantagrel, V., Lefeber, D. J., Ng, B. G., et al., 2010.
SRD5A3 is required for converting polyprenol to
dolichol and is mutated in a congenital glycosylation
disorder. Cell, 142(2), 203-217.
Grabińska, K. and Palamarczyk, G., 2002. Dolichol
biosynthesis in the yeast Saccharomyces cerevisiae: an
insight into the regulatory role of farnesyl diphosphate
synthase. FEMS yeast research, 2(3), 259-265.
Gründahl, J. E. H., Guan, Z., Rust, S., et al., 2012. Life
with too much polyprenol: polyprenol reductase
deficiency. Molecular Genetics and Metabolism,
105(4), 642-651.
Illian, D.N., Basyuni, M., Wati, R. and Hasibuan, P.A.Z.,
2018. Polyisoprenoids from Avicennia marina and
Avicennia lanata inhibit WiDr cells proliferation.
Pharmacognosy Magazine, 14(58), 513.
Ishiguro, T., Morita-Fujimura, Y., Shidoji, Y. and Sagami,
H., 2014. Dolichol biosynthesis: The occurrence of
epoxy dolichol in skipjack tuna liver. Biochemical and
biophysical research communications, 453(2), 277-
281.
Jozwiak, A., Gutkowska, M., Gawarecka, K., et al., 2015.
Polyprenol Reductase2 deficiency is lethal in
Arabidopsis due to male sterility. The Plant Cell,
27(12), 3336-3353.
Rosenwald, A. G., Stanley, P., McLachlan, K. R., & Krag,
S. S. (1993). Mutants in dolichol synthesis: conversion
of polyprenol to dolichol appears to be a rate-limiting
step in dolichol synthesis. Glycobiology, 3(5), 481-
488.
Sagami, H., Swiezewska, E., and Shidoji, Y. 2018. The
history and recent advances in research of polyprenol
and its derivatives. Bioscience, Biotechnology, and
Biochemistry, 82(6), 947-955.
Sari, D.P., Basyuni, M., Hasibuan, P.A., Sumardi, S.,
Nuryawan, A. and Wati, R., 2018. Cytotoxic and
Antiproliferative Activity of Polyisoprenoids in
Seventeen Mangroves Species Against WiDr Colon
Cancer Cells. Asian Pacific Journal of Cancer
Prevention, 19(12), 3393-3400.
Skorupins-Tudek, K., Wojcik, J. and Swiezewska, E.,
2008. Polyisoprenoid alcohols- recent results of
structural studies. The Chemical Record, 8(1), 33-45.
Surmacz, L. and Swiezewska, E., 2011. Polyisoprenoids–
secondary metabolites or physiologically important
superlipids?. Biochemical and Biophysical Research
Communications, 407(4), 627-632.
Szkopinska, A., Swiezewska, E. and Rytka, J., 2006.
Interplay between the cis-prenyltransferases and
polyprenol reductase in the yeast Saccharomyces
cerevisiae. Biochimie, 88(3-4), 271-276.
Tao, R., Wang, C., Ye, J., Zhou, H., and Chen, H. 2016.
Polyprenols of Ginkgo biloba enhance antibacterial
activity of five classes of antibiotics. BioMed
Research International, 2016.
Plant Polyprenol Reductase in the Database
15
