
Remediation of Coastal Marine Sediment using Iron
Ahmad Seiar Y.
1,*
, Y. Nakamura
1
, T. Miyatuji
1
, Y. Hagino
2
, T. Kobayashi
2
,
Y. Shigeoka
2
and T. Inoue
3
1
Institute of Urban Innovation, Yokohama National University, 79-5 Tokiwadai, Hodogaya-ku, Yokohama, Japan
2
Tokyo Kyuei Co. Ltd. 3-1-15 Nihonbashi, Chuoh-ku, Tokyo, Japan
3
Environment Information Research Group, Port and Airport Research Institute, 3-1-1 Nagase, Yokosuka, Japan
Keywords: Sedimentary Sulfide-release, Iron, Iron Hydroxide, Anoxia, Laboratory Experiment.
Abstract: Laboratory experiments were conducted to evaluate the effectiveness of iron application to surface sediment
on the suppression of hydrogen sulfide release from sediments. By using sediments cores collected from
Mikawa Bay, Japan at every month from June to September 2017, incubation experiments were made for
three weeks under anoxic conditions with or without application of the iron containing compounds; the iron
oxide or iron hydroxide. The results revealed that both uses of the iron oxide and iron hydroxide significantly
reduced sulfide release flux from the sediment into the overlying water. Iron hydroxide was more effective
than iron oxide in the suppression of sulfide release, as concluded from 21
-
day of incubation. While, no
significant difference was observed among the control group after 21day incubation. Therefore, it can be
conclude that the application of iron to the sediment is a promising method to remediate contaminated
sediments in eutrophic water body.
1 INTRODUCTION
Sediment is the important habitat for organisms living
in the surface and into the bottom ground. It acts as
the storehouse of nutrients in aquatic ecosystems.
Eutrophication because of excess amount of nutrients
supply to a water body causes high productivity that
results in large amount of organic matters settle to the
sediments. As the excess organic material is left to be
decomposed, and if the amount of oxygen is
insufficient, decomposition processes continue due to
bacterial activities employing electron acceptors
other than oxygen, this results in the reduction of
sulfate, (Wang and Chapman, 1999; Levin et al.,
2009; 2002; Yakushev et al., 2007; Ueda, 2013), as
per equation,
SO
4
2-
+ Organic Matter (OM
)
.
→
H
2
S + CO
2
In the absence of dissolved oxygen (DO) and in the
presence of soluble Biological Oxygen Demand
(BOD), Desulfovibrio desulfuricans (SRB) and other
sulfate-reducing bacteria (SRB’s) convert the sulfate
ion to sulfide, which is highly toxic and fatal to
benthic organisms. However, the irons have capacity
to regulate the formation of sulfide by poisoning the
*
http://www.cvg.ynu.ac.jp/G2/member_e.html
redox sequence and to form insoluble iron sulfide and
pyrite compounds. The chemical equation showing
this process is
keeping these points in view, for marine
environmental remediation, we aim to propose a
method for improving the sediment environment and
conduct an elution experiment using an undisturbed
sediment core added with various iron materials in
laboratory experiments to precipitate hydrogen
sulfide over a long period of time.
2 MATERIALS AND METHODS
Figure 1: A schematic view of the experimental apparatus.
Y., A., Nakamura, Y., Miyatuji, T., Hagino, Y., Kobayashi, T., Shigeoka, Y. and Inoue, T.
Remediation of Coastal Marine Sediment using Iron.
DOI: 10.5220/0007756303350339
In Proceedings of the 5th International Conference on Geographical Information Systems Theory, Applications and Management (GISTAM 2019), pages 335-339
ISBN: 978-989-758-371-1
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
335
2.1 Sampling Locations
Intact sediment cores were taken from a fix point in
the innermost part of Mikawa Bay, Japan, in order to
evaluate the suppression effects of iron application to
surface sediment on sulfide release rates. Mikawa
Bay is a eutrophic coastal embayment’s in which
seasonal density stratification and associated hypoxic
condition in the bottom water develop, in general,
from June to September. In this study, sediment core
samples were collected with acrylic pipe whose inner
diameter of 10 cm and length of 50 cm, every month
from June to September 2017. Temperature, salinity,
DO, and turbidity were measured at every sampling
occasions. All sampled sediment cores were
immediately transferred to a laboratory to conduct
sulfide release experiments with or without iron
application to the surface sediment.
2.2 Laboratory Experiments
Total 6 (for experiments in June, July and August) or
9 (September) core samples were selected for
incubation experiment. In the laboratory, the
overlying water of each core was replaced with
deoxygenated filtered seawater. For iron application
cores, predetermined amount of iron compounds were
applied to the surface of the sediment. Cores were
then sealed by a top cap to keep anoxic condition
during the course of the incubation period. DO meter
to check anoxic condition and a stirrer to circulate the
overlying water were also installed to a lid of pipe for
each core. Cores were then incubated into a container
keeping the same temperature of each the in-situ
conditions. Bottom water temperature for June, July,
August, and September experiments were 20.3, 21.7,
25.7 and 24.0 degree in Celsius, respectively.
The experiment was conducted with total four
kind of treatments (Reference core A, core B with
iron oxide applied to the surface, Unused core C, and
core D added with iron hydroxide, which was
performed only in September). In addition, each
experimental treatment was performed in triplicate
except the treatment B in June. Table 1 shows the
amount of iron compounds applied for each
experiment. Note that 5 g of iron oxide and 5.6 g of
iron hydroxide are equivalent to the same Fe amount
of 3.5 g.
The incubation experiments continued for three
weeks. Water samples were collected to measure the
dissolved sulfide and dissolved iron concentrations in
the overlying water at appropriate time intervals
during the incubation.
Table 1: List of treatments of the release experiment.
Treatments
Iron
compounds
Amount of iron compounds
applied [g]
June July Aug. Sept.
A: Control
Reference-1 0 0 0 0
Reference-2 0 0 0 0
Reference-3 0 0 0 0
B: Iron
oxide
addition
Fe
2
O
3
-1 0.41 5 5 5
Fe
2
O
3
-2 0.85 5 5 5
Fe
2
O
3
-3 1.61 5 5 5
C:
Experiment
unused
Only use in chemical analysis and preparative
D: Iron
hydroxide
addition
Fe (OH)
3
-1 - - - 5.6
Fe (OH)
3
-2 - - - 5.6
Fe (OH)
3
-3 - - - 5.6
2.3 Chemical Analysis
Dissolved sulfide was analysed by the methylene blue
method. In this method, a sulfide colouring reagent
comprising iron chloride III (FeCl
3
.6H
2
O) and N, N-
dimethyl-p-phenylenediamine sulphate dissolved in 6
M HCl solution were added into the sample for
analysis. The absorbance of the solution was
measured with a spectrophotometer at a wavelength
of 667 nm. Dissolved divalent iron concentration was
also analysed by the phenanthroline method.
Sediment quality was analysed after completion
of the experiment. Sediments were sliced to 1.5 cm
intervals, and water content, loss in ignition, TOC,
COD, sulfide, TN, TP, T-Fe, and T-Mn were analysed
for each sediment samples. Sediment pore water was
obtained by squeezing over a 0.45 µm filter, then
dissolved-sulfide concentrations were measured in
pore waters. A part of the collected sediment samples
was also used to analyze the hydrogen ion
concentration index (pH), oxidation-reduction
potential.
The data were analyzed using one-way analysis of
variance (ANOVA) at 0.05% level of significance
with the SPSS package (version 23 IBM).
3 RESULTS AND DISCUSSIONS
3.1 Dissolved Sulfide Concentration in
the Overlying Water
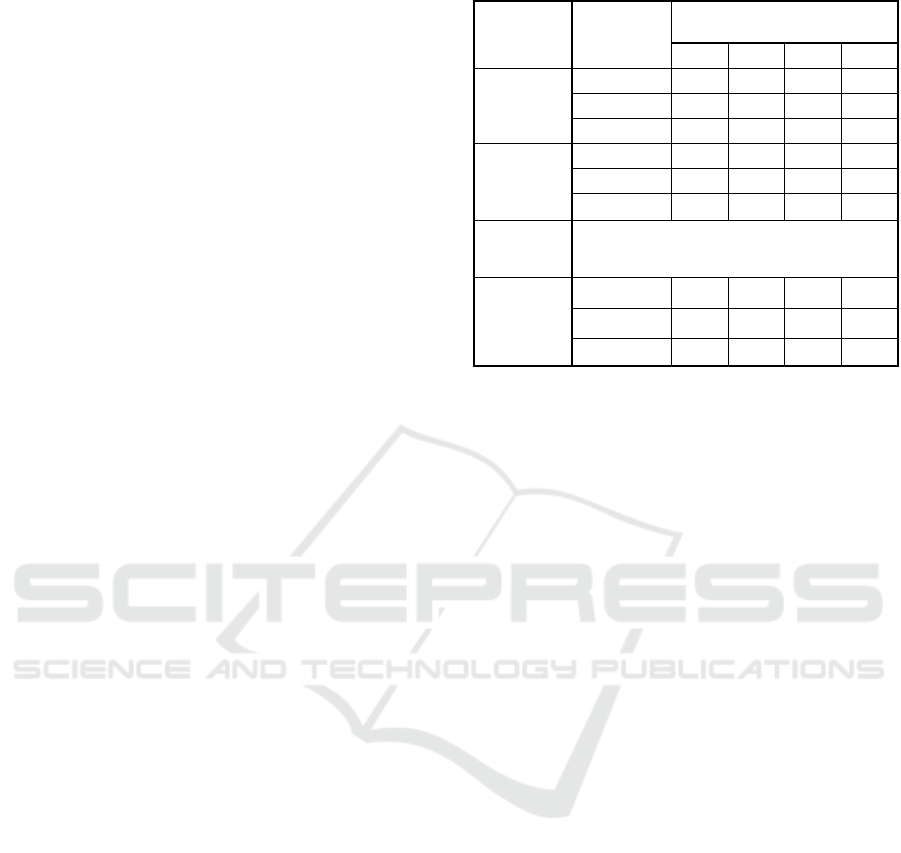
Temporal changes of dissolved sulfide concentrations
in the overlying water in each treatment are shown in
Figure. 2 (a), (b), (c), and (d) for the experiments
ONM-CozD 2019 - Special Session on Observations and Numerical Modeling of the Coastal Ocean Zone Dynamics
336
conducted in June, July, August and September,
respectively. For every cases, the concentration of
dissolved sulfide in the overlying water
monotonically increased related to sediment
remediation in all the cores.
Result for the experiments in June shows
relatively lower release of the dissolved sulfide into
the overlying water even in the control cases (A1, A2,
and A3). Order of the final concentration of dissolved
sulfide for B-1, B-2, and B-3 did not follow the
application amount of iron. Additionally, the final
concentration for cases of the application of iron
(Group B) showed no statistically significant
difference from the control case (Group A).
Therefore, for later experiments we used larger
amount of iron compounds for triplicate sediment
cores.
Table 2: Analysis of variance for reading comprehension of
the studied variables in the sediment.
Parameter Periods F Value P Value Result
Dissolved
sulfide
concentrations
in the overlying
water
June 3.845
0
.568
The result is not
significant at p < .05.
July and
August
6.643 0.011
The result is significant
at p < .05.
September 8.924 0 .000
The result is significant
at p < .01.
Dissolved
sulfide increase
rate
(mg/m
2
/day)
June 0.064 0.804
The result is not
significant at p < .05
July 0.891 0.367
The result is not
significant at p < .05
August 5.515 0.407
The result is significant
at p < .05.
September 9.401 0.002
The result is significant
at p < .01.
(a)
(b)
(c)
(d)
Figure 2: Temporal changes in dissolved sulfide
concentration in the overlying water for (a) June, (b) July,
(c) August, and (d) September experiments.
Remediation of Coastal Marine Sediment using Iron
337
Results obtained from cores for July, August and
September show a significant difference (p<0.05)
between the two treatment groups. Based on data
obtained in July, for example, the final concentration
of dissolved sulfide in the control cores (A-2, A-3, A-
1) were 44.4, 40.6, and 31.9 mg/L, respectively.
Whereas the final concentrations in the iron
treatments core (B-2, B-3, B-1) indicated 9.4, 12.5,
and 14.6 mg/L, respectively. These results showed
remarkably lower values compared to the control
cases as shown Figure 2 (b). The same tendency was
observed for the third August experiments.
In the last experiment in September, iron
hydroxide was also added to treatment groups. The
final concentration of dissolved sulfide in the
overlying water for iron hydroxide core (D-2, D-1, D-
3) indicates 0.4, 3.8, and 10.7 mg/L, respectively, as
shown in Figure 2 (d). These values were much
smaller than iron oxide application core (B-3, B-1, B-
2), in which those values were 23.2, 25.2, and 29.5
mg/L. The dissolved sulfide concentrations were
quite high in the control core (A-3, A-1, A-2) with
values of 73.6, 49.6, and 43.4 mg/L, respectively.
Although the averaged final concentration was
highest in the control case (A) in September, it was
lowest in the iron hydroxide application (D) This
suggests the relatively higher effectiveness of the iron
hydroxide for the suppression of sulfide release. The
lag time to appear significant increase in 5 mm
dissolved sulfide concentration was longest in June.
More than five days were necessary even in the
control case. The lag time became gradually shorter
in the later experiments. Especially in September, no
apparent lab time was observed.
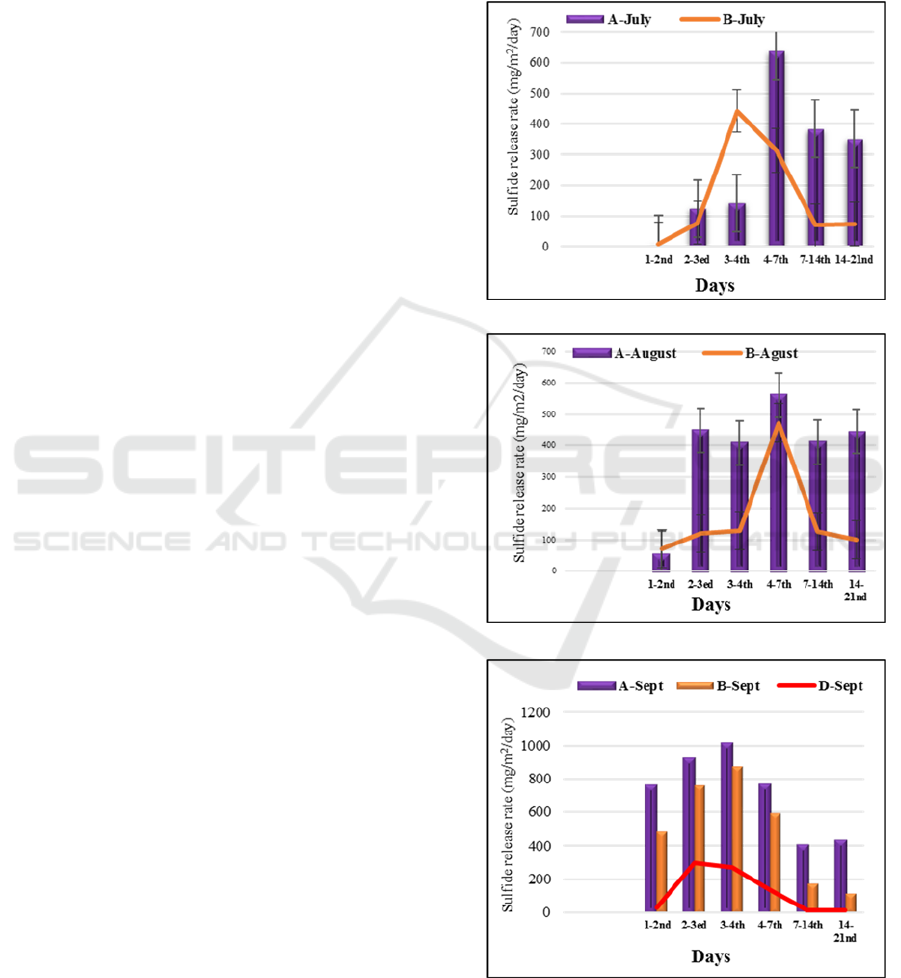
3.2 Dissolved Sulfide Release Rate
One of the practically inmportant parameters is the
release rate of dissolved sulfide from the sediment
under anoxic conditions. Averaged release rates
calculated for each time interval of the experimtns are
shown in Figure 3(a), (b), and (c), for July, August,
and September experiments.
In July and August experiments, the release rate
of dissolved sulfide in the control cores (A-1, A-2, A-
3) in turn ranged from 2 to 556 mg/m
2
/day and 8 to
637 “mg m
2
d
-1
”. However, in the experiment with
iron material added, it ranged from 1 to 521
mg/m
2
/day and 8 to 422 mg/m
2
/day.
Results of September experiment demonstrated
that the release rate as well as dussolved sulfide
concentrations in the overlying water were
significantly low with iron hydroxide core (D-2, D-1,
D-3) ranging from 1 to 269 mg/m
2
/day. As shown in
Figure 3(c), the second lowest value of the release
rate of dissolved sulfide were obtained from core (B-
3, B-1, B-2) were 116 to 845 mg/m
2
/day,
respectively. The release rate were quite high in the
control core (A-3, A-1, A-2) with values ranged from
409 to 1,014 mg/m
2
/day.
(a)
(b)
(c)
Figure 3: Release rate of dissolved sulfide for (a) July, (b)
August, and (c) September experiments.
ONM-CozD 2019 - Special Session on Observations and Numerical Modeling of the Coastal Ocean Zone Dynamics
338
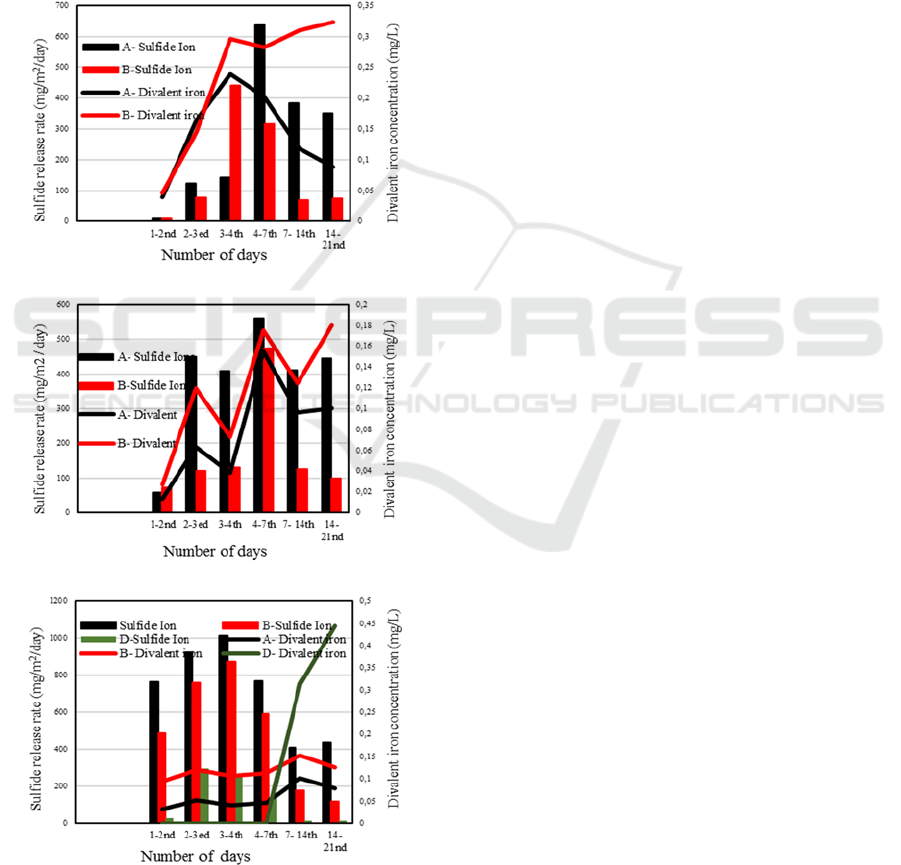
3.3 Sulfide Release Rate and Iron
Concentration
Figure 4 shows comparison between sulfide erlease
rates and divalent iron concentrations for July,
August and September experiments. The divalent
iron can react with dissolved sulfide to form
particulate iron sulfide, which will precipite to the
sediment. Such reaction may under-estimate the
release rates of dissolved sulfide. However, the
concentration range of divalent iron is relatively low
in these experiments.
(a)
(b)
(c)
Figure 4: Comparison of sulfide release rates and divalent
iron concentrations for (a) July, (b) August, and (c)
September experiments.
Further quantitative analysis on this point would
be necessary for further arguments.
4 CONCLUSIONS
The results revealed that both uses of the iron and
iron-hydroxide significantly reduced sulfide release
flux from the sediment into the overlying water. After
the 21 days incubation, the average dissolved sulfides
concentration in the overlying water of treatment
group was significantly decrease (p = .0001). No
significant difference was observed between the
control group after 21 day incubation. Therefore, the
application of iron to the sediment is a promising
method to remediate contaminated sediments in
eutrophic water body.
REFERENCES
Ueda, K., 2013. Modeling of dissolved oxygen
concentration recovery in water bodies and application
to hypoxic water bodies. World Envi., vol. 3, no. 2, pp.
52-59.
Levin, L. A., Ekau, W., Gooday, A. J., Jorissen, F.,
Middelburg, J. J., Naqvi, W., Neira, C., Rabalais, N. N.,
and Zhang, J., 2009. Effects of natural and human-
induced hypoxia on coastal benthos. Biogeosciences
Discuss., vol. 6, pp. 3563–3654.
Yakushev, E. V., Pollehne, F., Jost, G., Kuznetsov, I.,
Schneider, B., and Umlauf, L., 2007. Analysis of the
water column oxic/anoxic interface in the Black and
Baltic seas with a numerical model. Marine Chemistry,
vol. 107, pp. 388–410.
Wang, F. and Chapman, P. M., 1999. Biological
Implications of Sulfide in Sediment – A Review
Focusing on Sediment Toxicity, Environ Toxicol
Chem., vol. 18, no. 11, pp. 2526-2532.
Remediation of Coastal Marine Sediment using Iron
339
