
Design and Characterization of Synthetic Biodegradable Films for
Soft Tissue Engineering
Sofia Ribeiro
1,2 a
, Emanuel M. Fernandes
4,5 b
, Manuela E. Gomes
4,5 c
, Rui L. Reis
4,5 d
,
Yves Bayon
1 e
and Dimitrios I. Zeugolis
2,3 f
1
Institute Medtronic, Sofradim Production, Trevoux, France
2
Regenerative, Modular & Developmental Engineering Laboratory (REMODEL),
National University of Ireland Galway (NUI Galway), Galway, Ireland
3
Science Foundation Ireland (SFI) Centre for Research in Medical Devices (CÚRAM),
National University of Ireland Galway (NUI Galway), Ireland
4
3B’s Research Group – Biomaterials, Biodegradables and Biomimetics, University of Minho Headquarters of the
European Institute of Excellence on Tissue Engineering and Regenerative Medicine, Guimarães, Portugal
5
ICVS/3B’s – PT Government Associate Laboratory, Braga/Guimarães, Portugal
1 RESEARCH PROBLEM
To repair soft tissue, it is vital to ensure that the
biomaterial is able to mimic the complex elasticity
and topography of the native tissue. Huge efforts have
been invested into the development and design of
appropriate elastomeric biomaterials to match the
tissue of choice. However most of past studies have
used non-degradable polymers as substrates. In order
to obtain an optimal tissue engineered approach it is
vital to study the effect of biodegradable polymers.
The goal of the present study is to characterize
extensively a set of biodegradable polymeric micro-
grooved films and to assess its effect on cell adhesion,
morphology and phenotype through optimal substrate
rigidities.
2 OUTLINE OF OBJECTIVES
Production of biodegradable polymeric films with a
range of different stiffness.
Characterization of the physico-chemical and
mechanical properties of the polymeric films.
Assessment of the impact of substrate rigidity on
cell adhesion, proliferation and differentiation
potential.
a
https://orcid.org/0000-0002-0265-0416
b
https://orcid.org/0000-0002-4296-2529
c
https://orcid.org/0000-0002-2036-6291
d
https://orcid.org/0000-0002-4295-6129
e
https://orcid.org/0000-0002-0232-3079
f
https://orcid.org/0000-0002-7599-5191
Optimization of imprinting of micro-sized
grooves in the polymeric films.
3 STATE OF THE ART
Current surgical interventions are based on tissue
grafts; synthetic/natural biomaterials; direct cell
injections; and combinations of cells and a carrier
system. However, preclinical and clinical trials
revealed that tissue grafts are characterised by
delayed remodelling and substandard mechanical
function (Zeugolis et al., 2011); natural/synthetic
biomaterial-based substitution yields thinner and
weaker neotissue (Zeugolis, Chan and Pandit, 2011);
direct cell injections offer little control over localised
retention and distribution of the injected cell
suspensions, leading to scattered therapeutic
efficiency; and the presence of the carrier in the
cell/carrier system hinders normal tissue remodelling
and function (Abbah et al., 2014). All in all, current
surgical repairs do not restore soft tissue function,
imposing the need for new functional and clinically
relevant/viable regeneration strategies.
Research efforts have been directed towards
reconstruction of more functional in vitro
microenvironments using biopolymers (Gomes et al.,
2017) with optimized surface topography
(Vermeulen et al., 2019) and substrate stiffness (Li et
28
Ribeiro, S., Fernandes, E., Gomes, M., Reis, R., Bayon, Y. and Zeugolis, D.
Design and Characterization of Synthetic Biodegradable Films for Soft Tissue Engineering.
In Doctoral Consortium (BIOSTEC 2019), pages 28-32
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
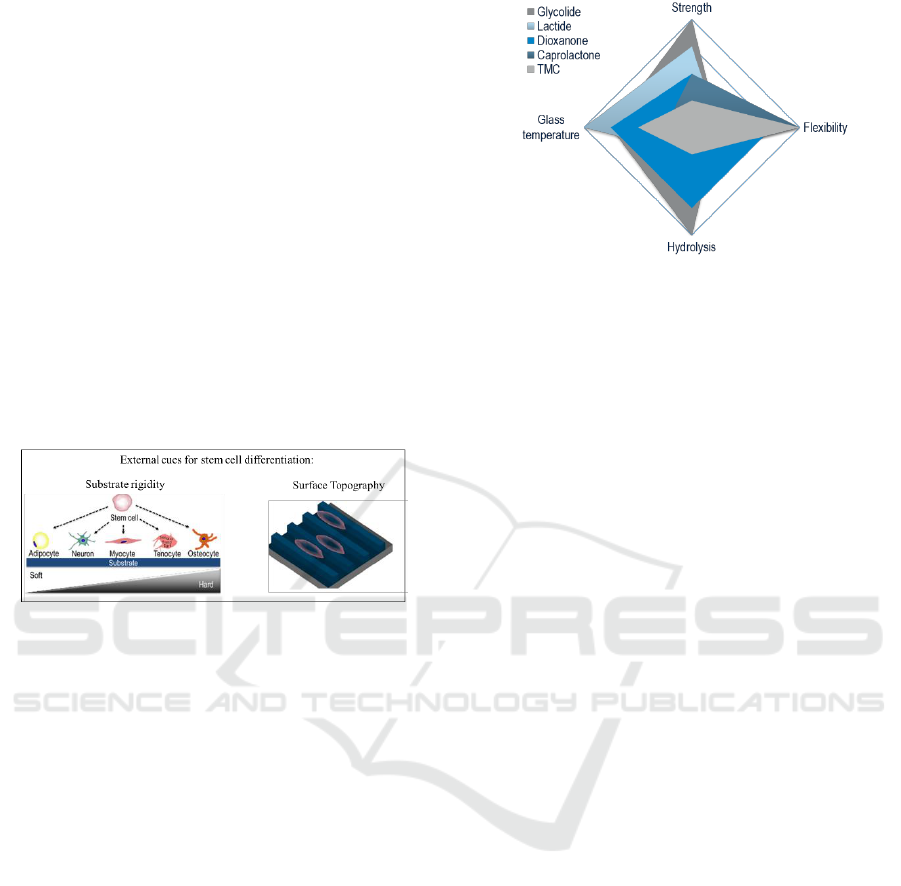
al, 2013) as means to control cellular growth, motility
and phenotype maintenance (Figure 1). The rationale
of using topographical features as means to control
cellular functions are based on the fact that
fundamental cellular substrata, the basement
membranes, are not smooth structures, but are
covered with the intertwined fibrillar meshwork of
the ECM (Jin et al., 2018). Similarly, rigidity plays a
crucial role in cell motility in vivo, as cells move from
regions of high ECM compliance to more stiff regions
(durotaxis) and in vitro studies have demonstrated
that stem cells commit to phenotype with extreme
sensitivity to elasticity; soft substrates that mimic
brain are neurogenic, whilst rigid substrates that
mimic bone are osteogenic (Engler et al., 2006; Lv et
al., 2015). Thus, it has been postulated that in vitro
recapitulation of physiological tissue topography and
rigidity will enable clinical translation of cell-based
therapies.
Figure 1: Substrate rigidity and surface topography as
external cues for stem cell differentiation.
4 METHODOLOGY
4.1 Film Preparation
Absorbable polyesters made from different
combination of monomers, such as lactic acid,
glycolic acid, trimethylene carbonate, dioxanone & β-
caprolactone, were selected for their physico-
chemical intrinsic properties. Even though the
selected polymers have similar chemistry they show
different mechanical and degradation properties
(Figure 2).
Polymeric films were produced by compression
moulding using a thermal presser Carver 3856 CE.
The presser was heated close to the polymer melting
temperature. The polymer was placed between Teflon
sheets and metal sheets inside the presser for 5
minutes. Then the system was placed under pressure.
The sample was removed after gradually cooling
down the system.
Figure 2: Monomers selected (Glycolide, Lactide;
Dioxanone, Ɛ-Caprolactone and Trimethylene Carbonate
(TMC)) and the representation of their intrinsic properties.
4.2 Chemical Characterization
NMR technique monitors the residual solvent
concentration in films and the monomer content. The
samples were prepared by dissolving the polymers in
CDCL3 or TFA deuterated. 1H NMR spectra were
obtained at 400 MHz. Measurements were performed
on a Fourier 300 Bruker spectrometer.
Differential Scanning Calorimetry (DSC)
technique was used to assess the glass transition (Tg)
and melting temperature (Tm), variation of enthalpy
of the polymeric films, as well as the crystallinity
content.
The DSC equipment used, a DSC 1 Star System,
Mettler Toledo, was programmed to perform two
heating curves, with a cooling intermediated step. The
temperatures used for each condition were optimized.
The mass of the analysed sample was between 5 and
6 mg. The second heating curve was the reference for
determining the Tg temperature, Tm and percentage
of crystallinity.
FTIR measurements were obtained using ATR
technique with a Spectrum 100 FT-IR Spectrometer,
Perkin Elmer by averaging 32 scans over the range of
4000 cm-1 to 800 cm-1.
The wettability of the samples was characterized
by static water contact angle measurements using a
sessile drop method with an OCA15+ goniometer
(DataPhysics, Germany) under ambient conditions at
room temperature. A 3 µL drop of distilled water and
diiodomethane (CH2I2) were dropped via a motor-
driven syringe. The data presented was calculated
using the final averaged values. The values for
polarity of the surface and the surface tension were
obtained by the Owens-Wendt method.
Design and Characterization of Synthetic Biodegradable Films for Soft Tissue Engineering
29

4.3 Mechanical Properties
4.3.1 Dynamic Mechanical Analysis (DMA)
The viscoelastic measurements were performed using
a DMA Q800 from TA Instruments. The
measurements were carried out at 37ºC in wet
conditions. Samples were cut in rectangular shapes
with about 14.5 x 5.3 mm (l,w) and 0.2 mm thickness
and clamped in the DMA apparatus. The sample was
deformed at constant stress-amplitude (25 µm) over 3
different frequencies (0.1, 1 and 10 Hz).
4.3.2 Tensile Tests
Mechanical properties were assessed under uniaxial
tension, using a Zwick/Roell (Leominster,
Herefordshire, UK) Z005 testing machine, loaded
with a 10 N load cell, as has been described
previously.
The samples were pre-cut into a dog-bone shape,
as per ASTM D882−2010 guidelines. Prior to testing,
all samples were incubated overnight at room
temperature in PBS and tissue paper was used to
remove excess PBS. The samples thickness was
measured using digital callipers (Scienceware, Digi-
Max, Sigma-Aldrich, Ireland). The samples were
hand-tightened between the vertical grips, which
were set at 50 mm gauge length. Scaffolds that broke
at contact points with the grips were rejected from the
analysis. The extension rate was set at 5 mm/min. The
following definitions were used to calculate
mechanical data: stress at break was defined as the
load at failure divided by the original cross-sectional
area (engineering stress), strain at break was defined
as the increase in scaffold length required to cause
failure divided by the original length, and modulus
was defined as the linear region of the stress-strain
curve using a stress at 0.02 strain divided by 0.02.
4.4 Degradation Studies
Films were incubated at 37°C in PBS for up to three
months. After predetermined periods of time samples
were removed from the solution, rinsed with distilled
water and dried at 37°C for 48 hours. Membrane mass
was weighted, and the percentage of weight loss was
calculated following equation 1:
% weight loss = (m
i
– m
f
)/m
i
X 100
(1)
Where m
i
and m
f
are the initial and final mass of
the sample, respectively.
4.5 In Vitro Assessment
4.5.1 Human Bone Marrow Stem Cells
(hBMSCs) Isolation and Culture
BMSCs were isolated according to standard
protocols. Briefly, bone marrow was flushed from
femurs and the flush-out solution was thoroughly
resuspended in complete basal medium and passed
through a 70 µm cell strainer. Cells were washed in
PBS- and were subsequently plated in complete basal
medium. After 2 days in culture non-adherent cells
were removed by several washes in PBS- and
cultured to near confluence (approx. 80%). Cells were
trypsinised, pooled, and re-plated (passage 0). Cells
were subsequently passaged at approximately 70-
80% confluency and were never allowed to reach full
confluency. Cells at passage 2 to 4 were used for all
experiments.
4.5.2 hBMSCs Seeding on Polymeric Films
Prior to cell culture studies, the films were sterilized
by ethylene oxide. hBMSCs at passages 3–4 were
harvested from culture flasks using trypLE Express
(Thermo Fisher, USA). Cells were washed with PBS
and centrifuged at 1200 rpm for 5 minutes. The cell
pellet was resuspended in α-minimal essential
medium (αMEM) supplemented with 10% fetal
bovine serum (FBS) and 2mM GlutaMAX and the
cells were seeded at different concentrations
described below onto the polymeric films, previously
placed into wells of a 24 well plate. The cells were
then cultured at 37 °C in a humidified atmosphere of
5% CO
2
. Subsequently, a drop of 100 μl of the cell
solution was seeded on top of the films and the cells
were allowed to attach at 37 °C, 5% CO2, 90%
humidity for 2,5 h prior to adding 900 μl complete
basal medium. The medium was changed every other
day.
4.5.3 Cell Adhesion
To evaluate the influence of the substrate stiffness in
cell morphology, 500 cell/cm
2
were seeded into the
polymeric films and incubated for 6 and 24 hours.
hBMSCs morphology was assessed by F-actin and
DAPI staining. Cells were fixed using a 10% formalin
solution for 30 minutes at 4°C. The samples were then
washed with PBS. For cell permeabilization it was
used a solution of 2% BSA in PBS. 4′,6-Diamidino-
2-phenylindole dihydrochloride (DAPI) and
Rhodamine B isothiocyanate were used to stain the
cell nuclei and F-actin filaments, respectively. The
samples were incubated with 1 mL of PBS containing
DCBIOSTEC 2019 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
30
5 µL of Rhodamine for 20 minutes at room
temperature and protected from light. After extensive
washing, samples were stained with 1µL or DAPI in
1 mL of PBS for 20 minutes at room temperature in
the dark. After staining the samples these were
washed again with PBS. The cells were visualized
using a fluorescence microscope.
4.5.4 Cell Proliferation
After each time point, the metabolic activity was
assessed by Alamarblue® assay. The samples were
placed in a new well-plate with 0.1M phosphate
buffer saline solution (PBS). 1 mL of 10%
Alamarblue® solution in PBS was added and a
negative control of Alamarblue® at 10% alone. The
cell culture plates were incubated for 3 hours at 37°C
at 5% CO2. A microplate read (Bio-Tek, USA) was
used to read the Optical Density (OD). Three
replicates of each condition were analysed.
To assess the hBMSCs proliferation seeded on
polymeric films and coverslips as control samples, a
fluorometric double-strand DNA quantification kit
(PicoGreen, Molecular Probes, Invitrogen) was used.
For this purpose, cell lysates were collected at 7, 14
and 21 days by transferring the samples into 1.5 mL
microtubes containing 1 mL of ultrapure water. The
samples were incubated for 1h at 37 °C in a water
bath, and next stored at -80 °C. Cell lysates and
standards (ranging from 0 to 2 mg mL-1) were
prepared and mixed with a PicoGreen solution in a
200:1 ratio and placed in an opaque 96-well plate.
The plate was incubated for 10 min in the dark, and
fluorescence was measured on a microplate ELISA
reader (Synergy HT, BioTek, USA) with excitation at
485/20 nm and emission at 528/20 nm. The DNA
values were calculated using a calibration curve.
4.5.5 Differentiation
For assessing the in vitro differentiation capacity,
cells were seeded onto polymeric films, as described
above, and were kept in complete basal medium for
48 h. Subsequently, the samples were placed in
appropriate differentiation media. The trilineage
differentiation capacity was confirmed after 21 days
in culture by and semi-quantitative qPCR.
4.6 Imprinting
Si master moulds with grooved topography (2x2 µm)
were fabricated via a photolithography process,
followed by reactive ion etching (RIE). Silicon
wafers (3.0 _3.0 cm
2
) were spin-coated with a
positive photoresist (S1813 PR, Shipley) and then
exposed using OAI Mask Aligner (Model MBA800).
Following photoresist development, the master
mould was etched by RIE (Oxford ICP etcher) using
CHF3 + SF6 ionised gas. The moulds were silanised
with 5 mM octadecyltrichlorosilane (OTS, Sigma
Aldrich, Ireland) solution to enable imprint release. A
thermal imprinting process was used to transfer the
master pattern into the polymeric films using a
nanoimprinter at optimized temperatures and
pressures. The imprinted gratings on polymer were
subsequently analysed by SEM and AFM. Non-
imprinted substrates were used as isotropic control
substrates.
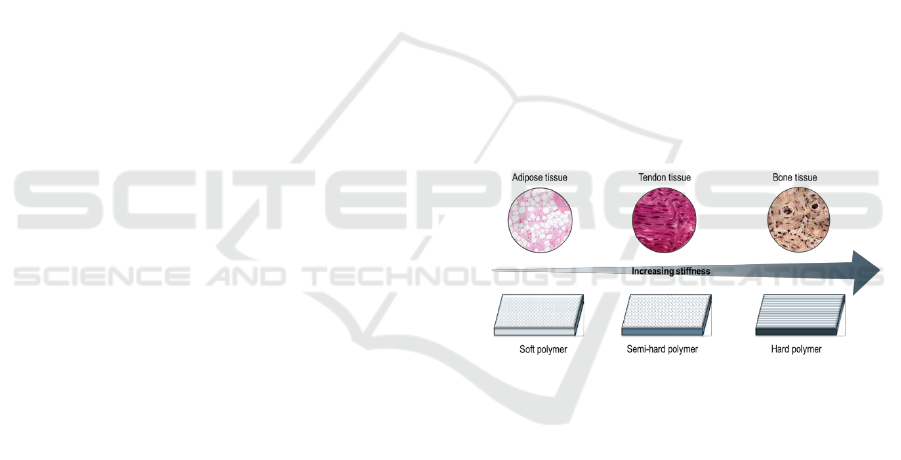
5 EXPECTED OUTCOME
It is expected to obtain polyester films with a range of
mechanical properties that will mimic the intrinsic
mechanical properties of the native tissue, such as
adipose, tenogenic and osteogenic tissue (Figure 3).
Chemical characterization and degradation studies
will provide information about the polyester prolife.
In vitro studies will shed some light on the impact of
the film’s stiffness to the cell fate.
Figure 3: Polyester films with increasing stiffness in order
to mimic adipose, tendon and bone tissue.
6 STAGE OF THE RESEARCH
It was possible to successfully produce polymeric
films using a large range of polyesters using a thermal
presser. Chemical analysis was used to trace the
chemical profile for each polymer. The selection
performed lead to materials with very distinct
profiles, regarding their crystallinity content and
degradation rate.
The mechanical properties of the materials were
analysed regarding at their macro level by DMA at
37°C in a PBS bath. The results show that the
developed films have a storage modulus ranging from
0.1 up to 2.6 GPa.
Design and Characterization of Synthetic Biodegradable Films for Soft Tissue Engineering
31

Biological assays showed good cell adhesion, cell
proliferation and cell viability. Cell morphology and
cluster formation were very different from one to
another polymer. The focal adhesion pattern has been
analysed as well, which means that the behaviour of
cells was strongly influenced by the nature of the
polymer and its associated stiffness, while other
parameters remained equal.
In the future, the combined effect of stiffness and
topography will be assessed on cell phenotype
maintenance.
This project is entering its final stage and is set to
finish in May 2019. As a disclosure, results cannot be
presented in details since 2 papers are under
preparation. In addition to this work, I co-authored
two book chapters and an additional review paper is
under preparation.
The results have been orally communicated at
these conventions: European Orthopaedic Research
Society, November 2018, Galway, Ireland; 4th
International Conference on Biomedical Polymers &
Polymeric Biomaterials, July 2018, Krakow, Poland;
and Future Investigators of Regenerative Medicine
(FIRM) Meeting, September 2017, Girona, Spain.
This work received funding from H2020-MSCA-
ITN-2015, Tendon Therapy Train Project (Grant
Agreement Number: 676338).
REFERENCES
Abbah, S. A., et al. 2014. Assessment of stem cell carriers
for tendon tissue engineering in pre-clinical models.
Stem Cell Research & Therapy. 5. 38.
Engler, A. J., et al. 2006. Matrix Elasticity Directs Stem
Cell Lineage Specification. Cell. 126. 677-689.
Gomes, M. E., et al. 2017. Tissue Engineering and
Regenerative Medicine: New Trends and Directions-A
Year in Review. Tissue Engineering Part B: Reviews.
23(3). 211-224.
Jin, G., et al. 2018. Electrospun three-dimensional aligned
nanofibrous scaffolds for tissue engineering. Materials
Science & Engineering C. 92. 995-1005.
Li, Z., et al. 2013. Differential regulation of stiffness,
topography, and dimension of substrates in rat
mesenchymal stem cells. Biomaterials. 34. 7616-7625.
Lv, H., et al. 2015. Mechanism of regulation of stem cell
differentiation by matrix stiffness. Stem Cell Research
& Therapy. 6:103.
Vermeulen, S., et al. 2019. Identification of topographical
architectures supporting the phenotype of rat tenocytes.
Acta Biomaterialia. 83. 277-290.
Zeugolis D.I., et al. 2011. Xenogenic tissues and
biomaterials for the skeletal system. In Comprehensive
Biomaterials. Volume 2. Oxford. Elsevier. 387–404.
Zeugolis D.I., Chan, J.C.Y., Pandit, A. 2011. Tendons:
engineering of functional tissues. In Tissue
Engineering. Berlin. Springer. 537–572.
DCBIOSTEC 2019 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
32
