
Genetic Diversity of Aspergillus flavus Isolated from Pepper
at North Sumatera
Kiki Nurtjahja
*1
, Cut Fatimah Zuhra
2
, Helmina Sembiring
2
, Betriana N. L. Gultom
1
1
Department of Biology, Universitas Sumatera Utara, Medan, Sumatera Utara, Indonesia 20155
2
Department of Chemistry, Universitas Sumatera Utara, Medan, Sumatera Utara, Indonesia 20155
Keywords: Aspergillus flavus, Genetic Diversity, Pepper, RAPD
Abstract: Aspergillus flavus is one of the postharvest fungi that often infects white and black pepper (Piper nigrum
L.). Fungal infection on pepper not only spoiled physically but also produce mycotoxins. The aim was to
study Aspergillus flavus toxigenicity and their genetic diversity on dried-stored white and black pepper sold
at traditional markets in North Sumatera. Fungal population was determined based on colony forming unit
per mililiter (cfu/mL) using serial dilutions and pour plated in dichloran 18% glycerol agar (DG18) medium.
Toxigenicity of A. flavus was determined culturally using coconut agar medium (CAM) 10%. Phylogenetic
between A. flavus strains were carried out by isolating genome of representative toxigenic and non-toxigenic
Aspergillus flavus strains according to the MiniKit Promega protocol and amplified using 10 Random
Amplified Polymorphic DNA (RAPD) primers. The amplified bands were scored and translated to be biner
data, then analyzed using Numerical Taxonomy and Multivariate System (NTSys) and clustered by
Unweighted Pair Group Method with Arithmatic Average Algorithm (UPGMA). Results showed that a total
of thirty one A. flavus strains were isolated. Based on toxigenicity determination found that twelve strains of
A. flavus were toxigenic (aflatoxin producers) and nineteen strains were non-toxigenic (non-aflatoxin
producers). Dendogram of similar bands was constructed and showed the highest similarity coefficient of A.
flavus strains was 0.84 (84%) which means that the strains were similar even though they were isolated
from different traditional markets.
1 INTRODUCTION
Pepper (Piper nigrum L.) i.e black and white
pepper, is one of spices that commoly produced by
tropical countries with high temperature, hummidity.
Most of the commodity is cultivated conventionally
and lack of good agricultural practices (Pickova et
al. (2020). Similar to crops, dried pepper is
susceptible contaminated by moulds. the infection
can cause spoiled physically and chemically, loss of
aroma, taste and contaminated by mycotoxins.
Fungal infection and aflatoxin contamination on
black and white pepper were previously studied. The
infection of pepper by Penicillium sp was reported
by Bokhari (2007) and Pitt and Hocking (2009).
Black and white pepper sold by retailer at
traditional markets were contaminated by
Aspergillus chevalieri, A. flavus, A. niger and A.
sydowi (Nurtjahja et al. (2019). Fungal infection
and mycotoxin contamination might occur during
preharvest and postharvest handling. Inappropriate
drying, storing might increase fungal population. As
a soil fungi, Aspergillus flavus contaminate crops in
field such as leaves, flowers and fruits crops
(Hererra et al. 2014). There are many strains and
genetic variability of Aspergillus flavus in field,
some of the strains are toxigen (aflatoxin producer)
and the other are non-toxigen (non-aflatoxin
produces) (Midorikawa et al. 2008; Ehrlich, 2014).
The genetic variability of Aspergillus flavus strains
on field was studied by Solorzano et al. (2014). The
objectives of the current study was to determine
genetic diversity of A. flavus strains isolated from
dried-stored black and white pepper sold at
traditional markets in Medan, North Sumatera.
Nurtjahja, K., Zuhra, C., Sembiring, H. and Gultom, B.
Genetic Diversity of Aspergillus Flavus Isolated from Pepper at North Sumatera.
DOI: 10.5220/0010766800002775
In Proceedings of the 1st International MIPAnet Conference on Science and Mathematics (IMC-SciMath 2019), pages 631-635
ISBN: 978-989-758-556-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
631

2 MATERIALS AND METHODS
2.1 Sample Collection
A total of 250 g samples of dried white and black
pepper were obtained from 5 retailers at five
traditional markets. Only intact seeds used in this
experiment. Each sample then was placed into a
sterile polyethylene bag and stored in a refrigerator
at ± 12°C for further use.
2.2 Determination Fungal Population
The population of A. flavus was determined by a
dilution and pour plated in dichloran 18% glycerol
agar (DG18) medium. Each sample was ground and
25 g were put into a 500 ml flask and suspended
with 250 ml of sterile distilled water and then
homogenized to obtain a 10
-1
suspension. Dilution
was carried out on 10
-2
, 10
-3
and 10
-4
. One ml of
dilution suspension was cultured on DG18 medium.
Each dilution was repeated 3 times. All plates were
incubated for 5 days (29
o
C). Population of A. flavus
per gram of pepper (cfu/g) was determined using the
formula:
Fungal population = Z (cfu/g)
X = volume of suspension transferred to each petri
dish (1 ml)
Y = dilution which gives the fungus colonies
separately
Z = average number of colonies of each fungal
species from 3 petri dishes
2.3 Fungal Identification
Each colony of A. flavus was cultured on potato
dextrose agar (PDA) medium then all of the isolates
were identified according to Pitt and Hocking
(2009).
2.4 Toxygenicity Determination of
Aspergillus flavus Strains
The toxigenicity of each A. flavus was determined
qualitatively by culturing in 10% coconut agar
medium (CAM) in petri dish (9 cm in diameter)
according to Lin and Dianese (1978). Toxigenic
strains was indicated by the presence of yellow
pigment at the reverse side of the medium.
2.5 Extraction Genome Aspergillus
flavus Strains
As much as 40 mg fungal mycelia in a microtube
containing 600 µl nuclei lytic was homogenised and
extracted using procedure Mini Kit (Promega,
Madison, WI, USA). Deoxyribonucleic acid
concentration obtained was determined using
nanophotometer (IMPLEN, Munich, Germany).
Electrophoresis of the DNA was conducted using
1.2% agarosa gel (SCIE-PLAS, Cambridge,
England) and stained by 1µl ethidium bromide
(EtBr) and visualise using Gel Doc (Uvitecc,
Cambridge, Serial) under UV light (303 nm).
2.6 Amplification of DNA and
PCR-RAPD
Amplification of DNA was conducted using 10
primers (Macrogen, Korea) as follows:
Primers
Nukleotides sequence
(5’ 3’)
OPA04 AATCGGGCTG
OPB10 CTGCTGGGAC
OPD10 GGTCTACACC
OPD20 ACCCGGTCAC
OPF10 GGCTGCAGAA
OPF13 GGAAGCTTGG
OPK20 GTGTCGCGAG
OPO20 ACACACGCTG
OPQ20 TCGCCCAGTC
OPT20 GACCAATGCC
PCR was performed as follows: a preincubation step
at 94
o
C for 10 minutes followed by 35 cycles of
denaturation at 94
o
C for 1 minute, annealing at 65
o
C
for 2 minutes, extension at 72
o
C for 2 minutes. The
PCR products were analyzed by electrophoresis
(SCIE-PLAS. Ltd, Cambridge, England) using 1.5%
agarose gels in 1 × TAE [40 mM Tris-acetate, 1 mM
EDTA (pH 8)]. Gels were stained with 0.5 µg µl
-1
ethidium bromide and visualized using Gel Doc
(Uvitec, Cambridge, Serial no. 13200263) under
UV light (303 nm). Polymorphic bands produced
were read and genetic diversity were analysed using
Numerical Taxonomy and Multivariate Analysis
System, version 2.1. (NTSYSpc21). Dendrogram
analysis was described by Unweighted Pair Group
Method with Arithmatic Average Algorithm
(UPGMA).
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
632
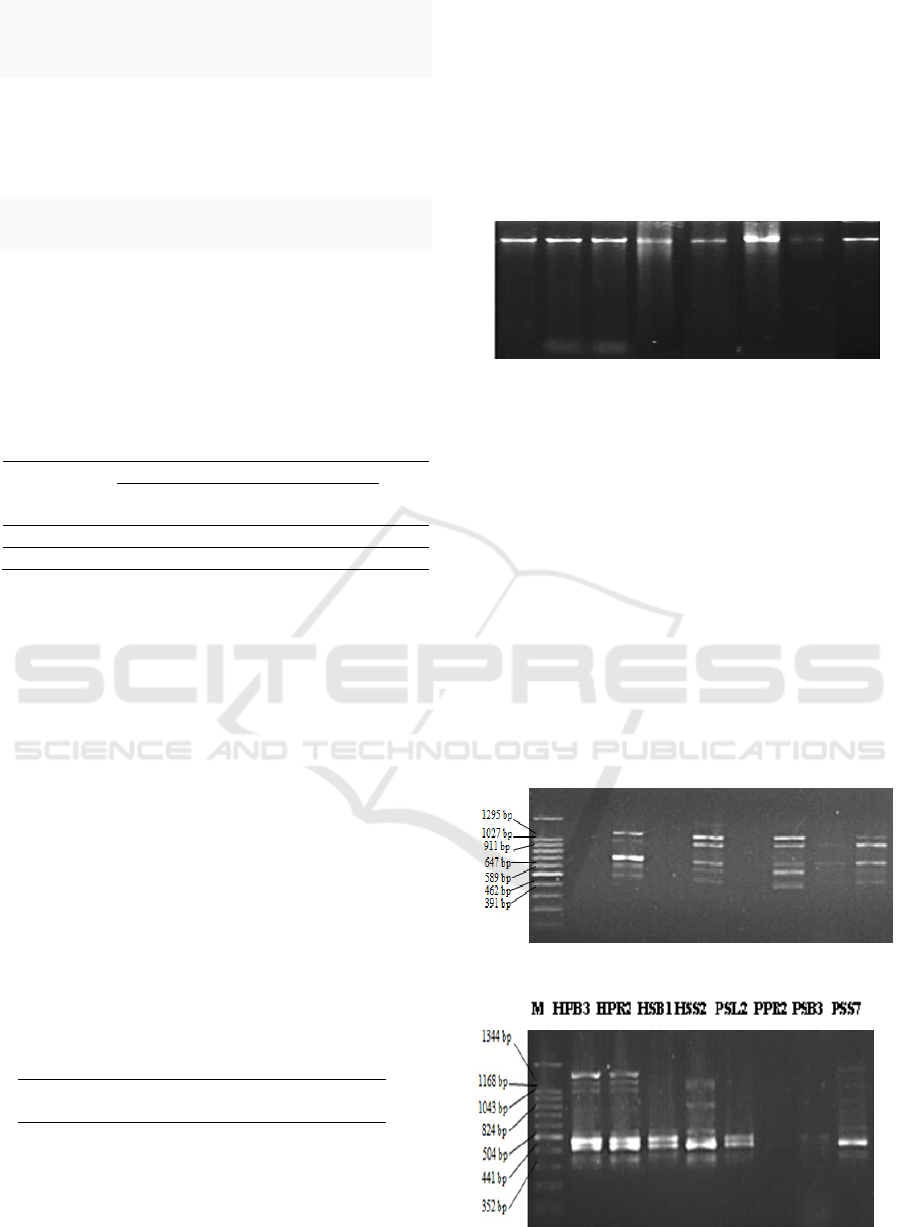
HPB3 HPR2 HSB1 HSS2 PSL2 PPR2 PSB3 PSS7
M HPB3 HPR2 HSB1 HSS2 PSL2 PPR2 PSB3 PSS7
3 RESULTS AND DISCUSSION
All samples, dried black and white pepper sold by
retailers at traditional markets, were stored in closed
jars.
3.1 Toxygenicity of Aspergillus flavus
Strains
Thirty one strains of A. flavus were successfully
isolated and the strains consisted of 10 strains
isolated from black pepper and 21 strains were
isolated from white pepper (Table 1).
Table 1.Total number of Aspergillus flavus strains isolated
from black and white pepper sold by retailers at traditional
markets
A
s
p
er
g
illus
f
lavus strains
total
toxigen non-toxigen
population
(cfu/ml)
b
lack
p
e
pp
e
r
3 7 0.5×10
3
10
white
p
e
pp
e
r
9 12 0.9×10
3
21
The population of A. flavus in black pepper was less
than that of white pepper, in addition to toxigenic
strains were less contaminated in black pepper than
that of white pepper. The presence of A. flavus in
black and white pepper indicate that the fungal
species was able to grow in dried pepper during
storage. We assumed that high fungal population
might occur during inappropriate storage or cross
contamination. Previous study by Leger et al. (2000)
stated that no specific host for A. flavus and they are
able to grow at minimum a
w
0.78 (Pii and Hocking,
2009). For phyllogenetic study, among of the 31
toxigenic and non-toxigenic A. flavus, only 8
genomes of representative traditional markets and
toxigenic and non-toxigenic A. flavus strains were
extracted as shown in Table 2.
Table 2. Representative toxigenic and non-toxigenic A.
flavus strains isolated from black and white pepper
obtained from each traditional market for phylogenetic
study
A. flavus
code
isolate sources toxigenicity
HPB3
b
lack peppe
r
toxigen
HPR2
b
lack
p
e
pp
e
r
toxi
g
en
HSB1
b
lack peppe
r
non-toxigen
HSB2
b
lack
p
e
pp
e
r
non-toxi
g
en
PSL2 white peppe
r
non-toxigen
PPR2 white
p
e
pp
e
r
toxi
g
en
PSB3 white peppe
r
non-toxigen
PSS7 white
p
e
pp
e
r
toxi
g
en
Genome extraction of 8 representative toxigenic and
non-toxigenic A. flavus strains is shown in Figure 1.
The purity each of the genome was between 1.6 to
1.8. Previous study by Sambrook et al. (1989)
reported that the purity of genome for molecular
study at ratio A
280
/A
280
was 1.8 to 2.0.
Figure 1. Electrophoresis of 8 strains of A. flavus
genomes
Amplification A. flavus genomes and PCR-RAPD
Amplification of genomes of 8 A. flavus strains
using 10 RAPD primers (OPA04, OPB10, OPD10,
OPD20, OPF10, OPF13, OPK20, OPO20, OPQ20,
OPT20) showed different bands, however, among 10
primers used, only 3 primers (OPD20, OPF10,
OPT20) were succesfully amplified and produce 7
bands with polymorphism 100%. The
electrophoresis of genome amplification using
primers were OPD20, OPF10, OPT20 is shown in
Figure 2.
Primer OPD20
Primer OPF 10
Genetic Diversity of Aspergillus Flavus Isolated from Pepper at North Sumatera
633

Primer OPT20
Figure 2. Electrophoresis of amplification of 8 genomes of
A. flavus strains using primers: OPD20, OPF10, and
OPT20. M= marker ladder (100 bp)
Random Amplified Polymorphic DNA (RAPD)
amplification in Figure 2 showed that each primer
has different bands and polymorphism percentage.
Primer OPF10 has the smallest band and the highest
on OPT20
3.2 Genetic Diversity Analysis of
A. flavus Strains
Based on matrix of on Table 4 showed that the
highest genetic distance with genetic similarity 0.21
occured on A. flavus strains: HSS2 and HPB3, PSL2
and HSS2, PPR2 and HPB3, PSB3 and HPR2.
Whereas, the lowest genetic distance with genetic
similarity 0.84 occured only in A. flavus strains
PSB3 and HSB1.
Table 4: Genetic similarity of A. flavus strains isolated
from black and white pepper sold by relailers at traditional
markets based on primers OPD20, OPF10 and OPT20
HPB3 HPR2 HSB1 HSS2 PSL2 PPR2 PSB3 PSS7
HPB3 1.00
HPR2 0.63 1.00
HSB1 0.63 0.26 1.00
HSS2
0.21 0.47 0.47 1.00
PSL2 0.78 0.52 0.73 0.21 1.00
PPR2
0.21 0.36 0.57 0.68 0.31 1.00
PSB3 0.57
0,21 0,84 0.52 0.68 0.52 1.00
PSS7 0.52 0.57 0.68 0.57 0.52 0.68 0.63 1.00
Dendrogram of all A. flavus strains are grouped in
one cluster with similarity 44% (Figure 3).
Figure 3. Dendrogram of A. flavus strains isolated from
dried black and white pepper sold by retailers at traditional
markets
Based on dendrogram in Figure 3 showed high
possibility that A. flavus contamination on dried
black and white pepper sold by retailers at 5
traditional markets took place at out of the markets,
it might occure at distribution chains. Even though,
their population may increase due to high relative
humidity during storage.
4 CONCLUSIONS
Black and white pepper sold at traditional markets
are contaminated by toxigenic and non-toxigenic A.
flavus. Cross contamination may increase during
storage, therefore, good hygienic practices
particularly on distribution chain also required to
reduce fungal infection and aflatoxin contamination.
ACKNOWLEDGEMENT
The research was funded by Universitas Sumatera
Utara, contract DRPM Reseach grant no.
152/UN5.2.3.1/PPM/KP-DRPM/2019
REFERENCES
Bokhari, F.M. 2007. Spices mycobiota and mycotoxins
available in Saudi Arabia and their abilities to inhibit
growth of some toxigenic fungi. Mycobiology
35(2):47-53.
Ehrlich, K. 2014. Non-aflatoxigenic Aspergillus flavus to
prevent aflatoxin contamination in crops: advantages
and limitations [review]. Frontier in Microbiol.
50(5):1-9.
Herrera, M., Herrera A., Ariňo A. 2014. Aflatoxin in
Food and Feed: Contamination, exposure, toxicology
and control. In: Aflatoxin Food Sources, Occurrence
and Toxicological Effects. Editor Adina G Faulkner.
New York (US): Nora Science.
Leger, R.J.S.T., Screen S.E., Shams-Pirzadeh B. 2000.
Lack of host specialization in Aspergillus flavus. Appl.
Environ. Microbiololy. 66(1):320-324.
Lin, M.T., Dianese J.C. 1976. A coconut-agar medium for
rapid detection of aflatoxin production by Aspergillus
spp. Phytopathology. 66:1466-1469.
Midorikawa, G.E.O., Pinheiro M.R.R., Vidigal B.S,,
Arruda M.C., Costa F.F., Pappas G.J., Ribeiro S.G.,
Freire F., Miller R.N.G. 2008. Characterization of
Aspergillus flavus strains from Brazilian Brazil nuts
and cashew by RAPD and ribosomal DNA analysis.
Letters in Applied Microbiology 47:12-18.
Nurtjahja, K., Zuhra C.F., Sembiring H, Bungsu A,
Simanullang J., Silalahi J,E., Gultom B.N.L, Sartini.
2019. Fungal contamination spices from Indonesia
red color= high similarity; yellow= low similarity
M HPB3 HPR2 HSB1 HSS2 PSL2 PPR2 PSB3 PSS7
IMC-SciMath 2019 - The International MIPAnet Conference on Science and Mathematics (IMC-SciMath)
634

with emphasis on Aspergillus flavus. Czech Journal of
Food Sciences 37(5): 338-344.
Pickova, D., Ostry V., Malir J., Toman J., Malir F. 2020.
A review on mycotoxins and microfungi in spices in
the light of the last five years. Toxins 12: 1-33.
Pitt,J.I., Hocking A.D. 2009. Fungi and Food Spoilage.
New York (US): Springer.
Sambrook, J., Russel D.W. 1989. Molecular Cloning: a
Laboratory Manual. Cold-Spring Harbor Laboratory
Press. New York.
Solorzano, C.D., Abbas H.K., Zablotowicz, Chang P.K.,
Jones W.A. 2014. Genetic variability of Aspergillus
flavus isolates from a Mississipi corn fields. The
Scientific World Journal Vol. 2014. Article ID
356059.
Genetic Diversity of Aspergillus Flavus Isolated from Pepper at North Sumatera
635
